NEONATAL SEPSIS and OTHER INFECTIONS
Definitions
Sepsis is defined as isolation of bacteria or other pathogenic organism from the blood of a baby with clinical signs. Proven sepsis in term infants is not common (1-2 per 1000 or 0.1%), but the diagnosis of suspected or clinical sepsis is made frequently. The incidence of proven sepsis is 20% in VLBW babies and up to 50% in the ELBW infant (less than 1000 g.). The high susceptibility of the developing fetus and newborn to sepsis is due to functional defects of both innate and adaptive immunity. These defects include immature cutaneous and mucosal barriers, low concentrations of T and B cells and lack of antigenic memory. Neonatal infections have been classified as early-onset (< 3 days of age), late-onset (>3-7 days of age) and very late onset (> 90 days of age). The definitions vary depending on the source of the literature, but the conclusions are generally comparable. Intrapartum prophylaxis for GBS has resulted in decreasing rates of early onset sepsis (EOS) for term infants. However, the incidence of non-GBS early onset sepsis is increasing for VLBW infants. Late onset neonatal sepsis (LOS) (from 8-90 days of life) affects two populations: healthy term infants in the community (which we will not discuss further) and preterm babies in the NICU - often referred to as nosocomial or hospital-acquired sepsis. Some experts consider infections acquired after 48-72 hours in the hospital to be hospital-acquired.
Clinical Presentation and Risk Factors
The signs of neonatal sepsis are extremely subtle in the newborn. Frequently, the nurse is the first to become suspicious and say "the baby is just not acting right." Clues include
- respiratory distress
- poor temperature control, particularly hypothermia
- poor feeding and poor suck
- jaundice
- abdominal distension with ileus
- vomiting
- lethargy
- irritability with tremors or seizures
- pallor or mottling, slow capillary refill, hypotension
- apnea
Factors which place a neonate at increased risk for EOS include:
- Maternal infection at the time of delivery (chorioamnionitis, urinary tract). This may include clinical evidence of chorioamnionitis (tender, painful uterus, sustained tachycardia), fever prepartum or postpartum, abnormal WBC count (>20,000 or <20,000 with "left" shift), urinary tract infection, or vaginal discharge which is foul-smelling or purulent
- Prematurity and low birth weight (LBW= <2500 g)
- Prolonged labor and fetal distress, i.e. fetal tachycardia (>160/minute)
- Meconium aspiration
- Instrumentation of the infant , i.e. placement of umbilical or urinary bladder catheters, intubation, etc.
- Prolonged rupture of membranes (PROM). BE AWARE: the abbreviation PROM is ambiguous. Obstetricians tend to define PROM as premature rupture of the membranes: greater than 4 hours of membrane rupture with no labor pattern established. Pediatricians tend to interpret PROM as prolonged rupture of the membranes, defined as 18 hours of more of membrane rupture prior to delivery of the baby. Preterm ROM occurs before 37 weeks gestational age.
- Abnormal color of amniotic fluid. Clear fluid is comforting; meconium-stained fluid is bothersome; purulent or malodorous material is definitely abnormal, particularly if the baby is also foul-smelling.
- Appearance of placenta and membranes. Foul-smelling placenta with thickened, opaque amniotic and chorionic membranes is indicative of chorioamnionitis. Funisitis indicates the umbilical cord is involved.
In VLBW infants, proven risk factors for LOS are:
- Birthweight <750 g
- Presence of central venous catheters
- Delayed enteral feeding
- Prolonged hyperalimentation
- Mechanical ventilation
- Complications of prematurity: patent ductus arteriosus, bronchopulmonary dysplasia, and necrotizing enterocolitis
Differential Diagnosis
Early newborn period (EOS)
- transient tachypnea of the newborn
- respiratory distress syndrome
- intracranial hemorrhage
- congenital cyanotic heart disease, especially if the baby is more than 24 hours old and has a ductal-dependent heart lesion
Late newborn-neonatal period (LOS)
- bowel obstruction
- necrotizing enterocolitis (NEC)
- metabolic disease
Based on consideration of all clinical factors, one must make a decision whether to observe the infant carefully, or culture and begin antibiotics. Preterm infants, especially those < 1500 g BW, should be treated more aggressively than a term baby.
Evaluation
The work‑up of an infant with suspected infection should include the following:
1. Examination with assessment of sensorium, tone, suck, and activity; review of vital signs
2. Review maternal history for risk factors and infections in the 2-3 weeks before delivery.
3. Complete blood count (CBC).
- A depressed (<9000) WBC count is suggestive of infection.
- An elevated ratio of immature to total neutrophils (> 0.2-0.4) is very suggestive of sepsis; an I/T ratio of >0.4 is clearly abnormal.
- Thrombocytopenia may be seen in neonatal infections, particularly if due to fungus; however, the study below showed that platelet count is normal in 82% of EOS cases.
A recent large multi-center study of CBC in sepsis at < 3 days (EOS, n=166,092) found that WBC<8800, ANC<1533 and I/T > 0.24 were significantly associated with EOS; however, no single CBC index was both sensitive and specific in predicting positive blood cultures. No association was found between elevated WBC and EOS. Hornik CP, Benjamin DK, Becker KC et al. Use of the Complete Blood Cell Count in Early-onset Neonatal Sepsis. Pediatr Infect Dis J. 2012; 31: 799-802.
4. Blood culture(s)
- After meticulous skin preparation, obtain 1 ml of blood from a peripheral vein.
- Two blood cultures should be obtained if the infant is >72 hours old.
5. Urinalysis (UA) and Urine culture in babies with presumed sepsis beyond 3 days of age. The genitourinary tract may serve as both a portal of entry for pathogens as well as a site of deposit for bacteria. The specimen should be obtained by bladder catheterization or suprapubic tap. But do not delay initiation of therapy while trying to obtain a satisfactory urine specimen.
6. Chest film. With chorioamnionitis due to prolonged rupture of membranes, intrauterine pneumonia is common. Pneumonia is frequently seen with GBS.
7. Rectal swab (if GI disturbances are present).
8. CSF culture. Because CNS involvement occurs in 25‑30% of babies with sepsis, CSF culture and examination for glucose, protein and cell count should be done unless the infant is clinically unstable, lumbar puncture is likely to cause further compromise, or initiation of antibiotic therapy will be delayed.
- CSF culture for enterovirus or HSV should be considered.
Cellular and chemical content of newborn CSF differs from that of older infants, as follows:
Examination of Cerebrospinal Fluid in Noninfected High-Risk Neonates*
|
Term Preterm ________________________________________________ White blood cell count (cells/mm3) Range 0-32 0-29 Mean 8.2 9.0 Protein (mg/dl) Range 20-170 65-150 Mean 90 115 Glucose (mg/dl) Range 23-119 24-63 Mean 52 50 Ratio of cerebrospinal fluid to blood glucose Range 44-248 55-105 Mean 81 74
|
|
* Modified from Sarff, L.D., Platt, L.H. and McCracken, G.H., Cerebrospinal fluid evaluation in neonates: Comparison of high risk infants with and without meningitis. J Pediatr. 88:473, 1976. |
Initial Therapy
In the first 72 hours of life, therapy should include ampicillin in combination with an aminoglycoside (Gentamicin). Initial therapy of suspected sepsis after 72 hours is usually vancomycin and gentamicin or cefotaxime.
The intravenous route should be used for therapy of meningitis or suspected sepsis. Duration of therapy for sepsis is 7‑10 days, and for neonatal meningitis, three weeks, or for two weeks after the spinal fluid becomes sterile ‑ whichever is longer. Dosages for commonly used antibiotics are summarized later in the chapter. Note that total dosage and intervals of administration vary with the postnatal age of the infant, the type of infection being treated, and in some instances the birth weight of the baby. (Source: Neofax, 2009).
Recent data from UTMB in the term infant and from the literature in preterm babies support the discontinuation of antibiotics when the blood culture is negative at 24-36 hours, IF ALL OF THE CONDITIONS BELOW ARE MET:
- Term newborn
- Ampicillin and Gentamicin are the antibiotics used
- Early onset sepsis (age 0-3
days)
- Blood culture is the only culture obtained, and
- Baby is asymptomatic at 24-36 hours
- For the preterm baby, consider stopping before 48h if two CBCs 12 hours apart have an I/T ratio less than 0.2
|
Murphy, Kara; Weiner, Joel. Use of Leukocyte Counts in Evaluation of Early-onset Neonatal Sepsis. Pediatr Infect Dis J 2012;31: 16-19. Background: Early-onset sepsis is a common diagnosis in neonatal intensive care units. Because of the low incidence, overtreatment is also common. Objective: To measure the sensitivity and negative predictive value of 2 serial white blood cell counts and a negative blood culture at 24 hours in predicting a noninfected neonate in the evaluation of early-onset sepsis. Methods: We performed a historical cohort study of neonates in the University of Massachusetts Newborn Nursery and neonatal intensive care unit born between 1999 and 2008 who had sepsis evaluations within the first 24 hours of life. Results: Three thousand two hundred thirteen patients were identified; 59 were excluded due to missing data. Of the 3154 included neonates, 1539 (49%) had 2 normal immature to total neutrophil (I:T) ratios and a negative blood culture at 24 hours. Normal I:T ratio was defined as <0.2. Two of these blood cultures showed growth of bacteria after 24 hours but were considered contaminants, and antibiotics were stopped at 48 hours. None of the 1539 neonates with normal I:T ratios was subsequently diagnosed with sepsis (negative predictive value 100%;95% confidence interval: 99.905% 100%). Conclusions: In this study, the combination of 2 serial normal I:T ratios and a negative blood culture at 24 hours in the evaluation of early-onset sepsis shortly after birth is indicative of a noninfected neonate. This suggests that antibiotics can safely be stopped at 24 hours in these neonates, which comprises approximately 50% of our study population. Key Words: early-onset sepsis, antibiotics, white blood cell count, I:T ratio |
Examples include the baby placed on antibiotics for what proved to be TTN, or the asymptomatic baby placed on antibiotics for a questionable CBC and GBS positive mother. If the baby is persistently symptomatic, preterm or there are other clinical concerns, continue the antibiotics.
Sepsis: Evaluation and Therapy in the Neonatal Nurseries Practice Guideline
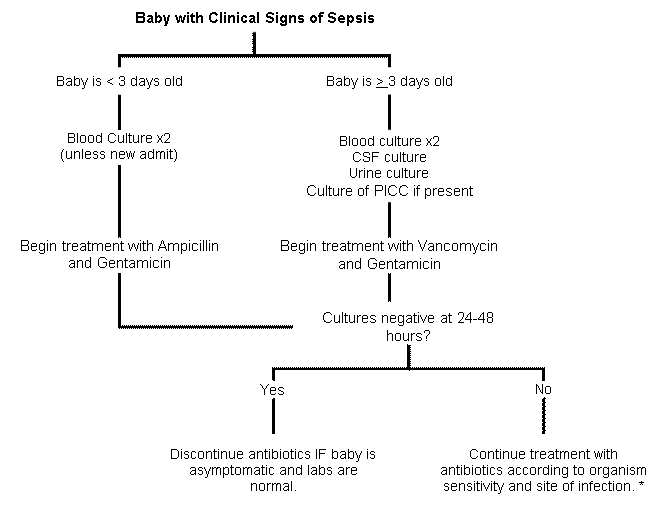
*Two positive blood cultures are required for the diagnosis of CONS sepsis.
Usual duration of antibiotic treatment:
- Sepsis or pneumonia: 7-10 days
- Meningitis: 2-3 weeks
- UTI: 7-10 days
Usual Pathogens in EOS
Group B b‑Hemolytic Streptococcus (GBS) Colonization and Disease
GBS are facultative diplococci which are subtyped into nine serotypes. Types Ia, Ib, II, III and V are the most common causes of neonatal disease. Type III is commonly associated with meningitis and late-onset GBS.
Pregnant women are colonized with GBS at rates of 15‑40%. Generally, they are asymptomatic but occasionally the organism causes postpartum infection with fever and sometimes systemic disease. Infection with GBS has been suggested as a cause of premature rupture of membranes, preterm labor, and postpartum fever. Higher colonization rates occur in women who are < 20 years old, primigravida, low socioeconomic status, or whose ethnicity is Hispanic, Caribbean, or African-American. Babies become colonized at birth or in utero or may become colonized nosocomially (neonate to neonate via hands of nursery personnel). It has been estimated that from 30‑70% of babies born to mothers with GBS will become colonized. However, early onset neonatal sepsis/meningitis or pneumonia has been found to occur in only about 1 baby per 100 colonized mothers.
Following is the algorithm used at UTMB for prevention of EOS in babies born to mothers with chorioamnionitis or colonized with GBS. It is modified slightly from the AAP/CDC guideline.(http://aapnews.aappublications.org/cgi/content/full/31/12/1,)
Perinatal Group B Streptococcus Infection Prevention Algorithm in the Well-appearing Baby
I. Mother GBS positive, and adequately treated, or treatment not indicated, specifically
-- Received penicillin, ampicillin, or cefazolin > 4 hours prior to delivery OR
-- No treatment and C-section delivery before onset of labor and with no membrane rupture prior to delivery:
Baby is term (> 37 weeks gestation):
Discharge at 24 hours (or with mother if C-section) IF access to medical care is readily available
Baby is preterm (<37 weeks gestation):
Observe in hospital for 48 hours (36 hours at discretion of the attending physician)
IIA. Mother GBS positive OR unknown with inadequate or no treatment prior to delivery and Baby is term (> 37 weeks)
Duration of membrane rupture <18h:
Observe in hospital for 48 hours (36 h at discretion of the attending physician)
Duration of membrane rupture >18h: CBC with differential at 6 hours of age
Observe in hospital for 48 hours (36 h at attending discretion)
IIB. Mother GBS positive OR unknown with inadequate or no treatment prior to delivery and Baby is preterm (<37 weeks)
CBC with differential at 6 hours of age
Observation in hospital for 48 hours (36 h at attending discretion)
III. Maternal chorioamnionitis, defined as her treatment with antibiotics during labor, regardless of GBS status or baby's gestation
CBC with differential at 6 hours of age
Observation in hospital for 48 hours (36 h at attending discretion)
IV. Maternal chorioamnionitis, defined as her treatment with antibiotics during labor, regardless of GBS status or baby's gestation, IF baby not well appearing
CBC with differential; Blood and CSF cultures (discuss with attending)
Antibiotic coverage
If baby appears sick, regardless of maternal GBS status, treat as appropriate cor clinical signs and symptoms.
Neonatal risk factors for early GBS infection include (each increases risk 5-35 fold): < 37 weeks gestation, ROM > 18 hours, mother with chorioamnionitis (chorio), bacteremia, or bacteriuria, multiple birth, heavy maternal colonization, < 20 years old.
In our ISCU, babies with GBS infections are placed on contact isolation however, this is beyond the recommendations of the AAP. Presently, there are no recommendations for treating colonized babies or hospital personnel since treatment has not been shown to reduce either maternal or neonatal disease. The current practice followed by UTMB obstetricians is to screen at 34-36 weeks for GBS, treat all mothers who are positive for GBS at delivery and treat all high risk patients in labor, i.e. those who have evidence of chorioamnionitis, are preterm, febrile, or have > 18 hours ROM.
Adequate treatment for maternal GBS is defined as one dose of penicillin, ampicillin, vancomycin or Cefazolin no less than 4 hours prior to delivery. Clindamycin is not considered to be adequate because of the high rate of resistance of GBS to this drug. However, at UTMB clindamycin is used only if GBS is sensitive; therefore, if the mother is treated with clindamycin and the GBS is documented to be sensitive, this is considered adequate treatmen
Treat GBS disease in the baby with ampicillin (200-300 mg/kg/day) or Penicillin G (250,000-450,000 units/kg/day), with the higher doses used for meningitis. Duration should be 10 days for sepsis, 14-21 days for meningitis, and 28 days for osteomyelitis. Bone and joint infections involving the hip or shoulder also require immediate surgical drainage, and the orthopedic surgeons should be involved as soon as the diagnosis is suspected.
Recurrent GBS disease occurs in 1-6% of cases. Repeat infection is treated similarly, but susceptibility testing is recommended. Recurrent GBS may be of a different strain. Rifampin is sometimes effective at eliminating mucus membrane colonization, but is less reliable than when used for meningococcemia prophylaxis.
Other Causes of Early Onset Neonatal Sepsis
Since the implementation of intrapartum antibiotic prophylaxis (IAP) against GBS, an increasing proportion of EOS cases are caused by E. coli and other gram-negative enteric organisms. Some, but not all, centers have reported increases in non-GBS EOS in preterm LBW infants.
Escherichia coli, particularly subtypes with the KI antigen, are the second most common organisms causing EOS. When E. coli sepsis/meningitis is suspected, a third-generation cephalosporin, such as Cefotaxime or Ceftazidime, should be added to the treatment regimen. Specific antibiotic therapy should be continued for 14 days in cases of E. coli bacteremia, and for 21 days in case of meningitis.
Next in frequency to GBS and E.coli are several gram positive organisms: Viridans streptococci (S. mitis, S. oralis, and S. sanguis), Group D Streptococcus (S. bovis), and Staphylococcus aureus. Most of the remaining infections in the United States are caused by Listeria monocytogenes or gram-negatives such as Klebsiella, Haemophilus influenzae, Enterobacter, Pseudomonas and Bacteroides fragilis.
Listeria monocytogenes are gram-positive, β-hemolytic rods which cause severe disease in pregnant women, their fetuses, in newborns and in the immune-compromised. Like GBS, it may be associated with both early and last onset disease. Incidence is 2-13 per 100,000 live births. Listeriosis is most commonly associated with ingestion of contaminated foods such as cheeses, deli meat and hot dogs. Infection may cause mild illness in a pregnant woman, but may result in spontaneous abortion or preterm labor.
EOS with Listeria is treated with ampicillin and gentamicin for 14 days; meningitis is treated for 21 days. Serial LPs are recommended until the CSF is negative.
Pathogens Causing LOS
Coagulase-negative staphylococci (CONS) account for almost half of LOS cases. Although rarely fatal or causative of site-specific disease, CONS often causes clinical instability associated with temporary cessation of feeds, increased respiratory support, longer hospitalizations, and poorer overall outcome.
Staphylococcus aureus is distinguished from CONS by the production of coagulase and by the presence of protein A in the cell wall. S. aureus causes bacteremia, meningitis, cellulitis, omphalitis, osteomyelitis and arthritis. Methicillin-sensitive S. aureus infections are treated with Nafcillin or Oxacillin, while methicillin-resistant staph (MRSA) requires treatment with Vancomycin. Additional therapy for persistent infection includes Rifampin, Linezolid or Daptomycin.
Enterococci (formerly Group D strep) are encapsulated organisms which produce biofilm and slime. Most enterococcal disease is associated with indwelling catheters and has low mortality. The treatment requires combined therapy of an aminoglycoside and Vancomycin or ampicillin. Vancomycin resistant enterococci (VRE) are an emerging problem.
Gram-negative organisms: LOS caused by E.coli, Pseudomonas aeruginosa, or Enterobacter has a high mortality rate (40-75%). Treatment of any of these bacteria requires two antibiotics.
Systemic Fungal infections: Disseminated neonatal candidiasis is usually caused by C. albicans or C. parapsilosis. Lethargy, increased apnea, feeding intolerance and poor perfusion are the common, non-specific clinical features of systemic candidiasis. Although thrombocytopenia is a consistent feature, it may not be found at presentation. Treatment is Fluconazole (10-12 mg/kg/day) or Amphotericin B (0.5 to 1 mg/kg/day) for 7-14 days. Meningitis should be treated with Flucytosine (5-FC) or Fluconazole. Further evaluation includes ultrasonography of the brain and kidneys to rule out abscesses, an eye exam to rule out endophthalmitis and an echocardiogram if a central line is in place to rule out catheter vegetations associated with endocarditis.
Malassezia furfur is a lipophilic dermatophyte which contaminates intravenous lipid preparations and frequently colonizes neonatal skin. Treatment consists of removing the contaminated catheter. Amphotericin B is indicated if the infection persists.
Common Focal Infections
Focal skin infections such as cellulitis, pustulosis and omphalitis are usually caused by S. aureus.
Conjunctivitis refers to inflammation of the conjunctiva.
Gonococcal conjunctivitis presents with chemosis, lid edema and purulent exudates at 1-4 days of age. Gram stain and culture will confirm the diagnosis. Treatment is with ceftriaxone 25-50 mg/kg IV or IM (up to 125 mg). Babies should be evaluated for systemic disease.
Chlamydial conjunctivitis presents with inflammation, swelling and yellow discharge at 5-14 days of age. Diagnosis is made with ELISA or DNA probe testing of conjunctival scrapings. The treatment is erythromycin 40 mg/kg/day every 6 hours for 14 days. Babies should be evaluated for pneumonia.
Bacterial conjunctivitis, diagnosed by culture of eye exudates, may be caused by S. aureus, E. coli or H. influenza. Minor inflammation can be treated with erythromycin or gentamicin ophthalmic ointment. Severe cases may be associated with H. influenza or P. aeruginosa, and must be treated with parenteral antibiotics.
Herpes simplex virus may also cause conjunctivitis.
Pneumonia is a challenging diagnosis to make in the neonate. The typical clinical presentation may be indistinguishable from sepsis, and antibiotic treatment is similar. The usual patient is either a newborn in the first day of life with respiratory distress, or an older infant who is ventilator-dependent due to bronchopulmonary dysplasia. Most neonatal pneumonias are diffuse; focal opacifications on a chest radiograph are atypical, and should prompt a search for other causes. Respiratory management is discussed in the Pulmonary chapter.
Urinary tract infection may occur due to, or may cause, bacteremia. The incidence is higher in male infants, unlike older age groups. Gram negative organisms and enterococci are the most common causative organisms. Although urine culture is not needed in the work-up for EOS, it is very important in the evaluation of LOS. Diagnosis is made with urinalysis and a urine culture obtained by suprapubic bladder aspiration (SPA) or by catheterization. A positive urinalysis is defined as positive leukocyte esterase (LE) or microscopy positive for WBC or bacteria. Only urine obtained by SPA or catheterization is suitable for culture; a positive is defined as pure growth of more than 50,000 cfu/ml.
In the absence of other systemic disease, UTI is treated with antibiotics for 7-14 days. Prior to discharge, a renal ultrasound should be performed to rule out an anatomic cause of the UTI. VCUG should be performed after the second UTI or if renal ultrasound shows structural abnormality. It is more difficult to perform than renal ultrasound and is associated with complications such as bladder rupture. However, after the second UTI the risk of vesicoureteral reflux grade IV-V (hydronephrosis) increases markedly. (reference, UTI Clinical Practice Algorithm, Pediatrics, September 2011).
Prophylaxis for UTI, when appropriate, is Amoxicillin 10-20 mg/kg once daily. Depending upon the diagnosis, these patients should be seen by Urology or Nephrology after discharge.
Osteomyelitis and septic arthritis result either from hematogenous seeding in babies with bacteremia, or direct extension from a skin infection. Signs include localized erythema, swelling, pain on movement, or lack of spontaneous movement of the involved joint or extremity. The hip, knee and wrist are the usual joints involved. The femur, humerus, tibia, radius and maxilla are the bones most commonly affected.
S. aureus, GBS and gram-negative organisms are the typical causative organisms, so empiric treatment is with nafcillin, oxacillin, or vancomycin, and gentamicin.
Surgical drainage is often essential for infected joints, and orthopedic surgery should be consulted.
In the preterm baby, orthopedics should be consulted emergently because of the potential of irreversible damage to a septic joint. Duration of therapy is 3-4 weeks, ideally with an antibiotic known to be effective for the specific infecting organism.
Disability following osteomyelitis or septic arthritis can be significant due to the vulnerability of the growth plate at this age.
Commonly Used Antibiotics
AMPHOTERICIN B
Indications: Systemic fungal infection
Dose and Interval: 0.5 to 1 mg/kg every 24 hours IV infusion over 2-6 hours.
Comments: Mix in D5W (precipitates in saline); concentrations should not exceed 0.1 mg/ml. Line should be flushed with D5W only, prior to administration. Incompatible with almost everything.
Toxicity: Nephrotoxicity (decreases renal blood flow and GFR), hypokalemia, hyponatremia, RTA, vomiting, chills, fever, anemia, thrombocytopenia, phlebitis. Monitor CBC, electrolytes, urine output, BUN and creatinine.
AMPICILLIN
Indications: Initial treatment of neonatal sepsis and meningitis; gram positive organisms (except penicillin‑resistant Staph); Listeria, H. influenzae, some E. coli.
Dose and Intervals: 25-50 mg per dose by IV slow push over 15‑30 minute infusion or I.M.
|
Postconceptual Age (weeks) |
Postnatal Age (days) |
Interval (hours) |
|
<29 |
0 to 28 > 28 |
12 8 |
|
30 to 36 |
0 to 14 > 14 |
12 8 |
|
37 to 44 |
0 to 7 > 7 |
12 8 |
|
≥45 |
All |
6 |
Comments: Toxicity rare; very large doses may result in CNS excitation or seizures.
CEFOTAXIME OR CEFTAZIDIME
Indications: Gram-negatives (E. coli, Klebsiella, H. influenza, Serratia, Proteus). Ceftazidime is preferred if P aeruginosa is strongly suspected and other gram positive antibiotic coverage is used.
Gonococcal infections: Cefotaxime 25 mg/kg per dose IV over 30 minutes, or IM.
Gonococcal prophylaxis for maternal gonorrhea: 100 mg/kg Cefotaxime over 30 min. or 1M.
Dose and Administration:
For cefotaxime: 50 mg/kg per dose IV infusion by syringe pump over 30 minutes, or IM.
For ceftazidime: 30 mg/kg per dose.
|
Postconceptual Age (weeks) |
Postnatal Age (days) |
Interval (hours) |
|
<29 |
0 to 28 > 28 |
12 8 |
|
30 to 36 |
0 to 14 > 14 |
12 8 |
|
37 to 44 |
0 to 7 > 7 |
12 8 |
|
≥45 |
All |
6 (Cefotaxime) 8 (Ceftazidime) |
Comments: Very few significant side effects have been reported, but may include rash, phlebitis, diarrhea, leukopenia, granulocytopenia and eosinophilia.
CLINDAMYCIN
Indications: Treatment of bacteremia, NEC with possible perforation, and pulmonary or deep tissue infections caused by anaerobic bacteria and some gram-positive cocci. Clindamycin should not be used in the treatment of meningitis.
Dosing and Administration: 5 to 7.5 mg/kg per dose IV over 30 minutes, or PO.
|
Postconceptual Age (weeks) |
Postnatal Age (days) |
Interval (hours) |
|
<29 |
0 to 28 > 28 |
12 8 |
|
30 to 36 |
0 to 14 > 14 |
12 8 |
|
37 to 44 |
0 to 7 > 7 |
12 8 |
|
> 45 |
All |
6 |
Comments: Monitor GI and liver status. Increase dosing interval in patients with significant liver dysfunction. Pseudomembranous colitis is most serious adverse effect.
FLUCONAZOLE
Indications: Treatment of systemic infections and severe superficial mycoses caused by Candida species which are unresponsive to amphotericin B.
Dose and Administration:
Systemic Infections: 12 mg/kg loading dose, then 6 mg/kg per dose IV infusion by syringe pump over 30 minutes, or PO.
Prophylaxis: 3mg/kg/dose IV
Thrush: 6 mg/kg on Day 1, then 3 mg/kg per dose q 24 hours PO.
|
Postconceptional Age (weeks) |
Postnatal Age (days) |
Interval (hours) |
|
< 29 |
0 to 14 > 14 |
72 48 |
|
30 to 36 |
0 to 14 > 14 |
48 24 |
|
37 to 44 |
0 to 7 > 7 |
48 24 |
|
> 45 |
All |
24 |
Comments: Serum fluconazole concentrations are not routinely followed. Assess renal function, AST, and ALT. Interferes with metabolism of barbiturates and phenytoin, and possibly theophylline.
GENTAMICIN
Indications: Initial treatment of newborn sepsis; gram-negative infections (proven or suspected).
Dose and Administration:
|
Postconceptual Age (weeks) |
Postnatal Age (days |
Dose (mg/kg/dose) |
Interval (hours) |
|
< 29* |
0 to 7 8-28 >28 |
5 4 4 |
48 36 24 |
|
30 to 34 |
0 to 7 >7 |
4.5 4 |
36 24 |
|
> 35 |
All |
4 |
24 |
|
* or significant asphyxia, renal dysfunction, PDA, or treatment with indomethacin |
|||
Administer by IV infusion by syringe pump over 30 minutes. (Administer separately from penicillin-containing compounds if serum drug levels are planned.). IV is the preferred route.
Gentamicin may be administered IM, but its absorption and blood levels may be erratic, particularly in VLBW babies.
Comments: Toxicity includes nephrotoxicity associated with prolonged high troughs and ototoxicity associated with prolonged high peak serum concentration.
Serum levels in term babies (> 37 weeks at birth). If renal status is stable, levels are not needed if therapy is given for 48-72 hours. For babies on longer courses of gentamicin therapy, obtain pre and post levels at the third dose (see below) and check serum levels 5 hours after the dose of gentamicin on about day 7, and weekly thereafter. Spot levels should be <2 mcg/ml.
Serum levels should be obtained in preterm infants as follows:
With IV administration, obtain initial levels at 3rd dose. Obtain trough level just before administering the 3rd dose and the peak 30 minutes after the end of a 30 minute infusion. Obtain levels earlier if the patient's renal status is unstable.
With IM administration, obtain levels around the 3rd to 5th dose. Obtain a trough level just before administering the drug and a peak level one hour after the dose. Trough levels should be <2 mcg/ml and peak levels should be 4-8 mcg/ml and < 10 mcg/ml. If adjustment of the dose is needed (based on the initial peak and trough), redraw levels only if gentamicin therapy will continue for more than four more days.
PENICILLINS
Aqueous (Crystalline) Penicillin G: IM or IV
Indications and Dosages:
GBS sepsis: 200,000 units/kg/day
GBS meningitis: 450,000 units/kg /day
Bacteremia (other than GBS): 25,000-50,000 units/kg per dose
Meningitis (other than Group B Strep): 75,000-100,000 units per dose IV over 30 minutes.
Congenital Syphilis (with or without CNS disease): 50,000 units/kg given every 12 hours (100,000 u/kg/day), for the first 7 days, then given every 8 hours for 7 days.
Dosing intervals:
|
Postconceptional (weeks) |
Postnatal (days) |
Interval (hours) |
|
<29 |
0 -14 >14 |
18 12 |
|
30 to 36 |
0-1 > 14 |
12 8 |
|
37-44 |
0-7 > 7 |
12 8 |
|
≥45 |
Any |
6 |
Procaine Penicillin G
For Congenital syphilis: 50,000 units/kg/day IM every 24 hours for 10‑14 days.
Benzathine Penicillin G
For congenital syphilis: 50,000 units/kg (one dose) IM
Comments: Very well tolerated. Low toxicity.
VANCOMYCIN
Indications: Methicillin‑resistant S. aureus, coagulase‑negative staph.
Dose and Administration: Initial empirical dosing: 15 mg/kg/dose for meningitis and 10 mg/kg/dose for bacteremia. Obtain serum trough level at the 3rd dose, just before administering the dose. Maintain trough serum levels of 5-10 mcg/ml. When treating MRSA pneumonia, endocarditis or bone joint infections consider troughs of 15-20 mcg/ml. Peak levels are needed only when treating meningitis; the level is obtained 60 minutes after the 60 minute infusion and the target is 30-40 mcg/ml. If adjustment of the dose is needed, redraw a trough level if vancomycin therapy will continue for more than 4 more days. Give IV over 60 minutes.
Toxicity: Nephrotoxicity and ototoxicity, which is enhanced by aminoglycosides; rash and hypotension (red man syndrome), neutropenia, and phlebitis.
