The UTMB Human Research Protection Program (HRPP) and associated Institutional Review Board (IRB) strive to assist the research community on the application and review processes associated with human subjects research.
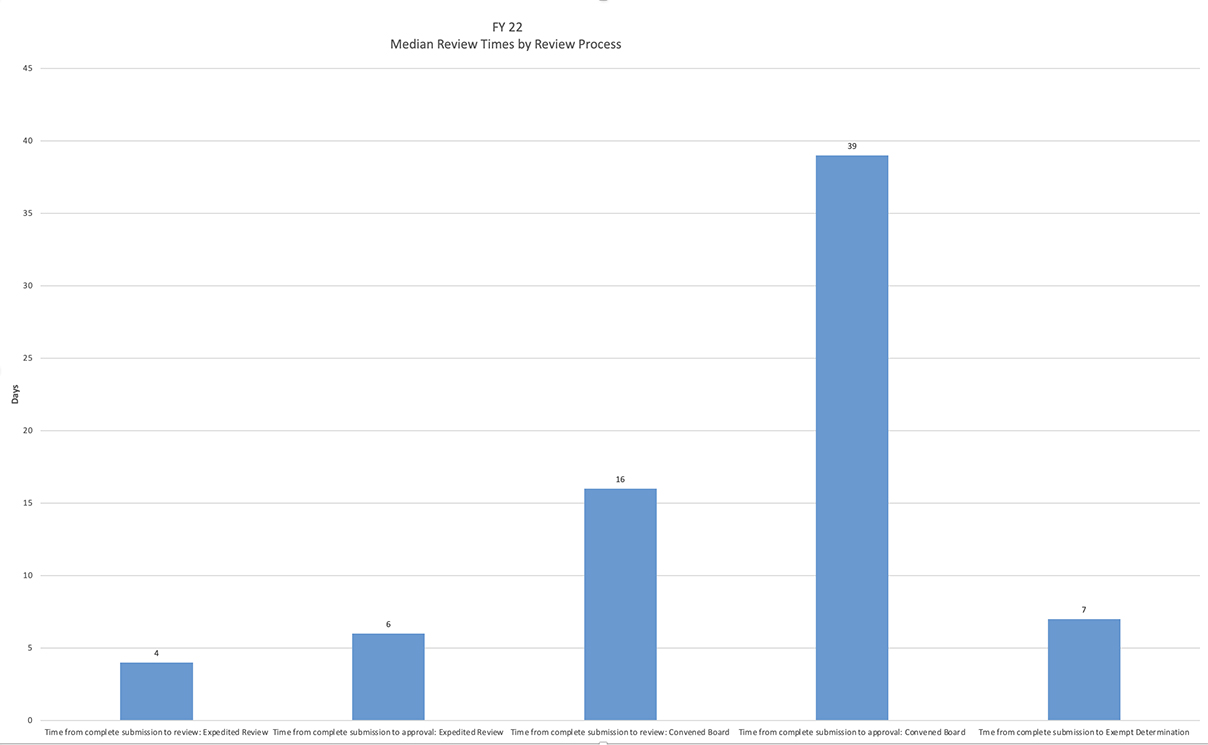
Review times are reported as median calendar days. A review begins when a completed new protocol submission is received by the IRB and ends when a final determination letter is issued by the IRB. These metrics are a snapshot. Protocols in pending status,
modifications, and renewals are not reflected here. Please keep in mind that processing times are affected by many factors including volume, response time, application information quality, staffing, and reviewer availability.
The processing includes all the following:
- Verification of personnel and training
- Pre-review by IRB staff
- Review by an IRB Chair/Vice Chair or designated staff member
- Response to revisions (both by the IRB and investigators)
- Processing the final decision and determination letter
A Complete submission means the IRB application is ready for review by a convened IRB Committee or expedited reviewer and contains the information needed to make the determinations outlined under the Federal regulations section 45CFR46.