Cyanotic Cardiac Lesions
D-Transposition of the Great Arteries (D-TGA)
More Info: Corrected Transposition of the Great Arteries (L-TGA)
Pathology
Both atrio-ventricular and ventricular arterial relationships are reversed. The right atrium is connected to a morphologic LV that is located on the right, and the left atrium is connected to a morphologic RV located on the left (ventricular inversion). The great arteries are also transposed, i.e. the aorta is connected to the morphologic RV on the left, and the pulmonary artery is connected to the morphologic LV on the right. The aortic valve is anterior and to the left of the pulmonary valve. Hemodynamically, both systemic and pulmonary circulations are in series. L-TGA is commonly associated with other CHD such as VSD (50%), pulmonary stenosis, left AV valve (tricuspid) insufficiency, complete AV block (AVB) and other arrhythmias.
L= levo, meaning 'left'
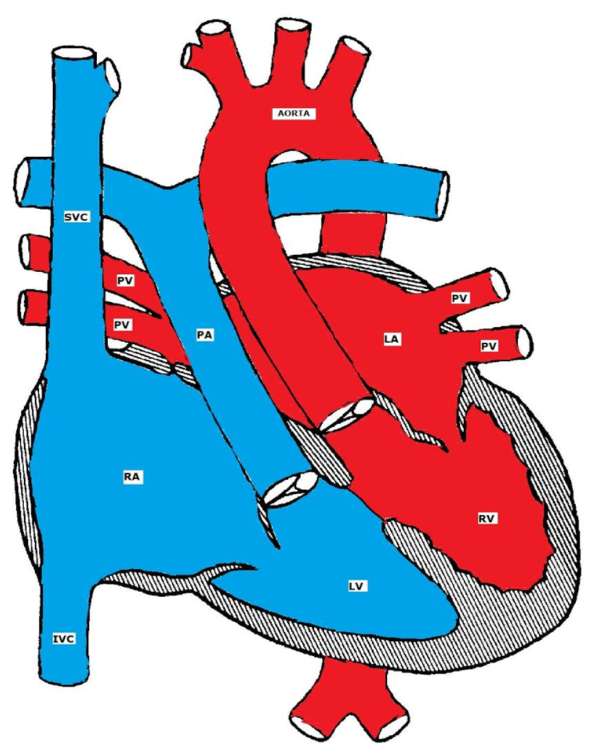
Corrected Transposition of the Great Arteries (L-TGA)
Making the diagnosis
- Patients with L-TGA are usually asymptomatic unless they have other CHD or arrhythmias.
- CHF may develop later as the systemic RV fails to pump against a high systemic vascular resistance.
- Signs and symptoms of associated CHD or arrhythmia may be present.
- On physical examination, the S2 is loud and single (the aorta is anterior to the PA).
- A holosystolic murmur at the LLSB may indicate left AV valve (tricuspid) insufficiency.
- EKG shows absent Q waves in left precordial leads and their presence in V4R (due to reversed septal depolarization), AV block, WPW or other arrhythmias may be present.
- CXR shows a straight left upper cardiac border formed by the ascending aorta.
- Segmental anatomy can be shown by echocardiography. Other associated CHD can also be detected.
Management
Medical: management of CHF and arrhythmias if present. Strenuous sports restriction is indicated to prevent strain of the systemic right ventricle.
Surgical: repair of associated CHD (VSD, PS, tricuspid valve replacement). Pacemaker implantation may be needed for AVB. Double switch procedure (arterial and atrial) has been tried to treat the failing RV. Heart transplantation may be considered in some cases.
Anatomy
In D-transposition of the great arteries (D-TGA), the aorta is positioned anterior and to the right of the pulmonary artery (instead of the normal right posterior relationship). The aorta is connected to the right ventricle and the pulmonary artery is connected to the left ventricle.
D=dextro, meaning 'right'
Although D-TGA is not the most common cyanotic CHD, it is the most common cardiac cause of neonatal cyanosis. It is more common in male infants of diabetic mothers who are usually large for gestational age (happy chubby blue boy).
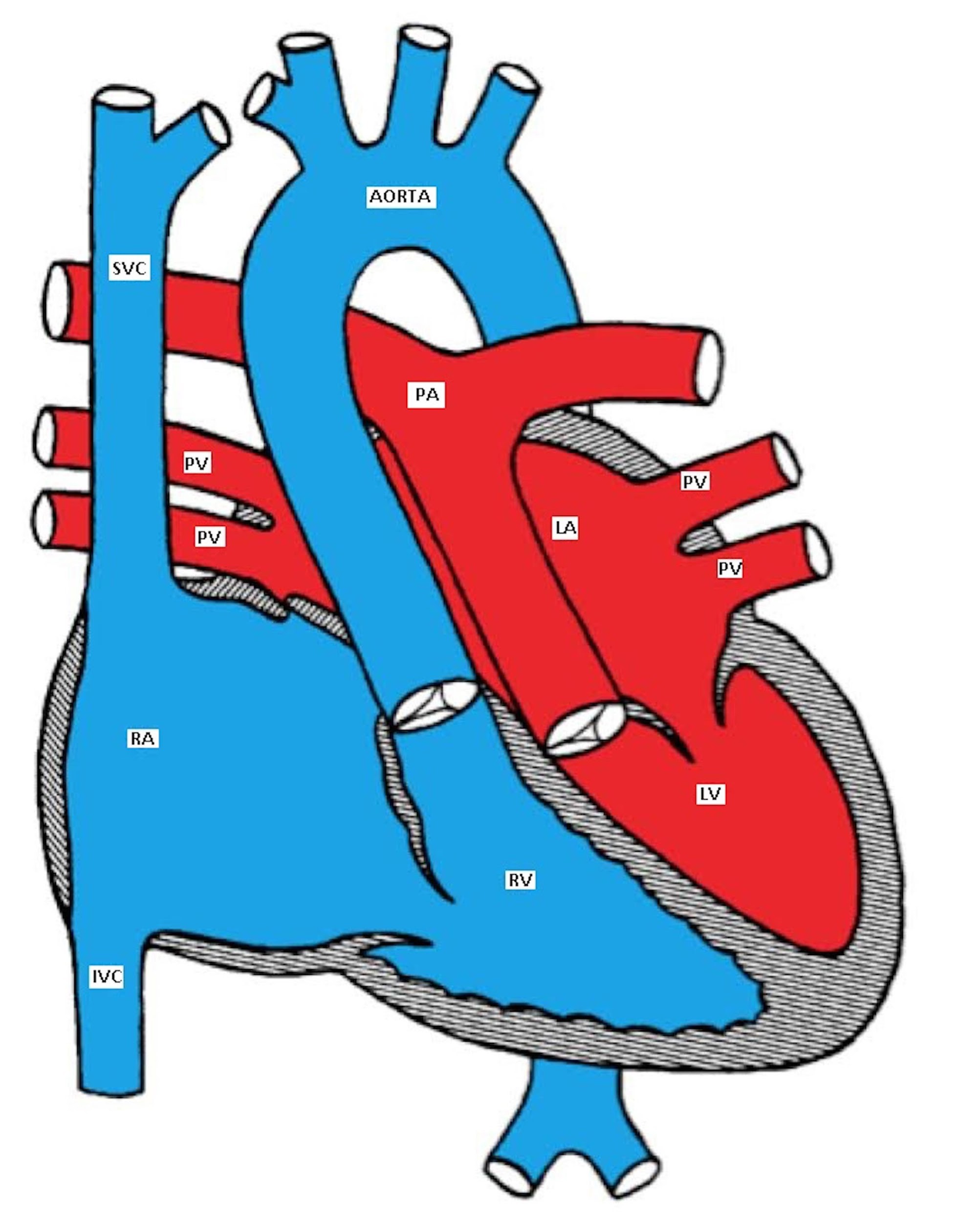
D-TGA
Pathophysiology
The blood runs in two parallel circulations (rather than the normal series circulation). This situation is incompatible with life unless there is mixing between the two circulations. The mixing may occur at the atrial level (ASD or PFO), ventricular level (VSD) or at the arterial level (PDA). The atrial level communication is the most important as a bidirectional shunt can easily be produced due to the low pressure difference between the two atria as compared to the ventricles or the great arteries.
Clinical Presentation
The physical examination is usually benign except for severe cyanosis and a single loud S2.
Making the Diagnosis
- EKG usually shows right axis deviation and right ventricular hypertrophy (RVH).
- CXR may show the characteristic egg on a string appearance (the great arteries lose their normal criss-cross relationship and the thymus may be hypoplastic).
- Echocardiogram is diagnostic of D-TGA. Echo is also important for identifying sites of communications between the two circulations and in delineating the coronary artery anatomy which is important in the surgical repair.
- Cardiac catheterization may be needed to perform atrial septostomy, (which could also be done under echo guidance) to allow mixing at the atrial level.
|
Features |
Right ventricle |
Left ventricle |
|
Shape |
Semi-lunar (crescent in cross section) |
Cone shaped (circular in cross section) |
|
Wall thickness |
Thinner (beyond the neonatal period) |
Thicker (beyond the neonatal period) |
|
AV valves |
Tricuspid |
Mitral |
|
Location of the AV valves |
Apically placed |
Basally placed |
|
Papillary muscles
|
Three, ill-developed |
Two, well developed |
|
Trabeculations |
Coarse |
Fine |
|
Ventricular Septum |
Trabeculated |
Smooth |
|
Moderator band |
Present |
Absent |
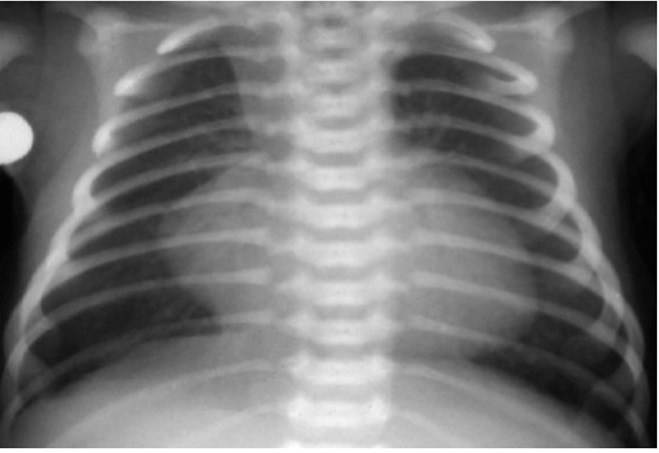
x-ray showing an egg on a string of D-TGA
Management
Medical management includes starting PGE1 to keep the ductus arteriosus open and treatment of metabolic acidosis if present.
Surgical repair options include:
Arterial switch (Jatene) procedure in which the great arteries are switched back to the corresponding ventricle and the coronary arteries are reimplanted in the neo-aorta.
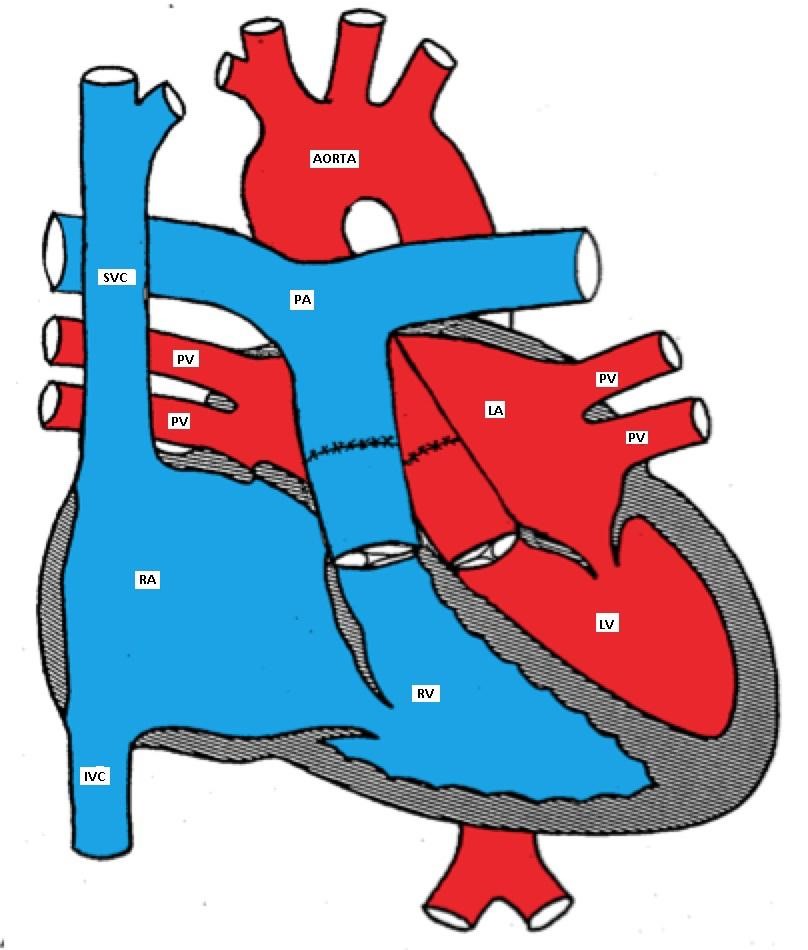
D-TGA after arterial switch surgery
Atrial switch (Senning or Mustard) procedure involves redirecting the blood flow at the atrial level so the pulmonary venous blood is baffled to the right ventricle (systemic ventricle) and the systemic venous blood is directed to the left ventricle (pulmonary ventricle). It is a less favorable surgery because of its numerous complications.
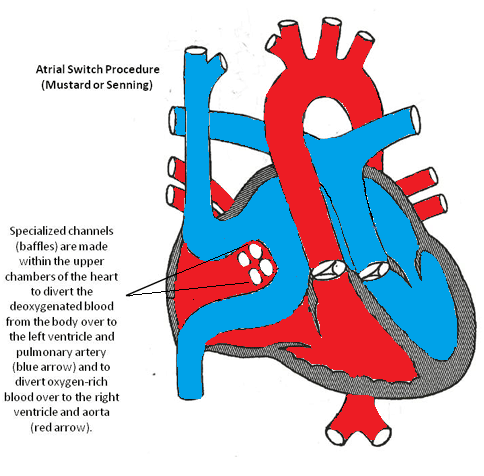
Atrial Switch Procedure
Persistent Truncus Arteriosus (TA)
Truncus arteriosus is sometimes associated with DiGeorge syndrome (deletion of chromosome 22q.11) which is characterized by hypocalcemia and reduced T-lymphocyte function. Extra-cardiac abnormalities are present in 25-40% of patients.
Anatomy
A single large trunk overrides a large VSD and gives rise to the coronary arteries, pulmonary arteries, and the brachiocephalic arteries.
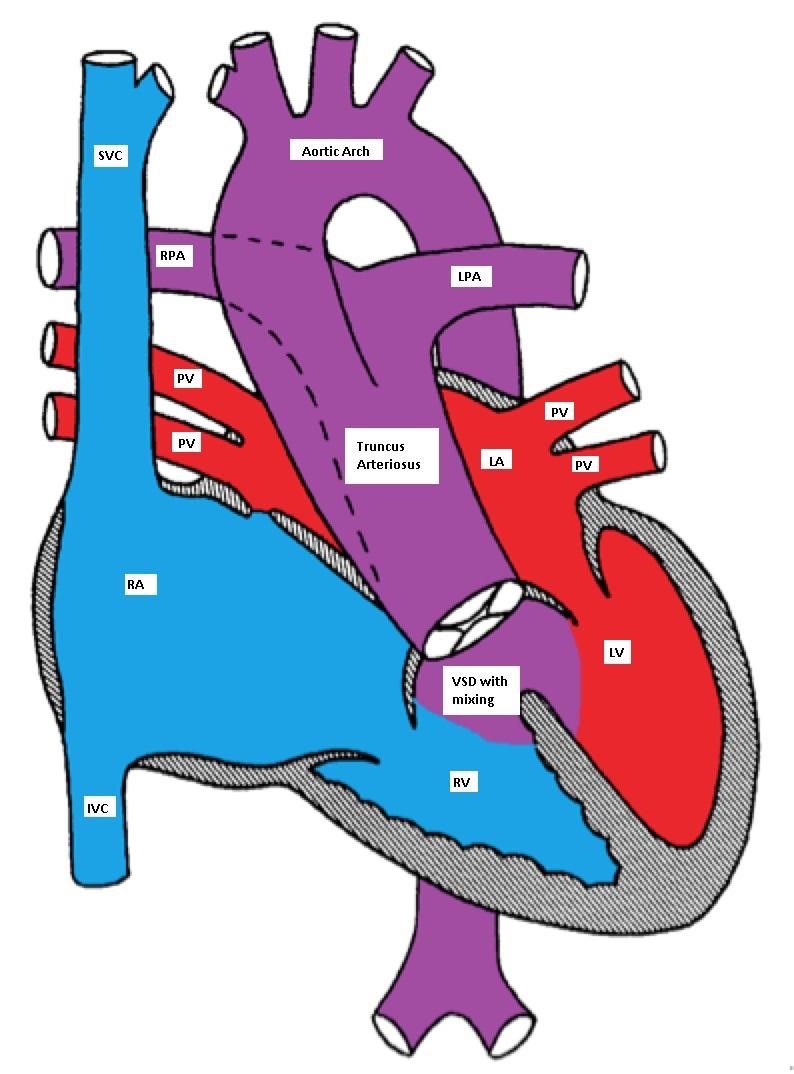
Truncus Arteriosus, with the main pulmonary artery arising from the common trunk
Pathophysiology
TA has a single ventricle physiology as the VSD is usually large (one ventricle pumps blood to both systemic and pulmonary circulations with complete mixing of blue and red blood). The truncal valve is frequently abnormal. In the neonatal period, the pulmonary vascular resistance is elevated and the pulmonary blood flow may be limited. This may cause mild and early cyanosis due to a right-to-left shunt. As the pulmonary vascular resistance drops, the pulmonary blood flow significantly increases causing CHF symptoms.
Clinical Presentation
Physical Examination: mild cyanosis may be detected along with a wide pulse pressure and cardiomegaly. A single S2 (one semilunar valve) and a systolic ejection murmur due to increased flow through the truncal valve may be heard. An apical diastolic murmur may be heard due to increased flow through the mitral valve (relative mitral stenosis). An early diastolic murmur may indicate truncal valve insufficiency.
Making the Diagnosis
- EKG may show biventricular hypertrophy.
- CXR shows increased pulmonary vascular markings and prominent ascending aorta. It may also be suggestive of right aortic arch (25% of cases).
- Echo is diagnostic and shows associated cardiac anomalies.
Management
Medical management of CHF may be tried until surgical repair can be performed.
Surgical repair may include:
- Early surgical correction is preferred. The VSD is closed so that the LV is connected to the truncus and the pulmonary artery is detached from the truncus and is connected to the right ventricle via a conduit.
- If early surgical repair cannot be done, banding of the pulmonary artery is performed to limit the blood flow to the lung and to control CHF.
Tetralogy of Fallot (TOF)
Tetralogy of Fallot is the most common cyanotic CHD (over 50% of all cases of cyanotic CHD; ~ 10% of all cases of CHD).
Anatomy
TOF has four abnormalities (hence the term 'tetralogy):
- Ventricular septal defect (mal-alignment)
- Overriding aorta
- Right ventricular outflow tract obstruction (RVOTO)
- Right ventricular hypertrophy (RVH)
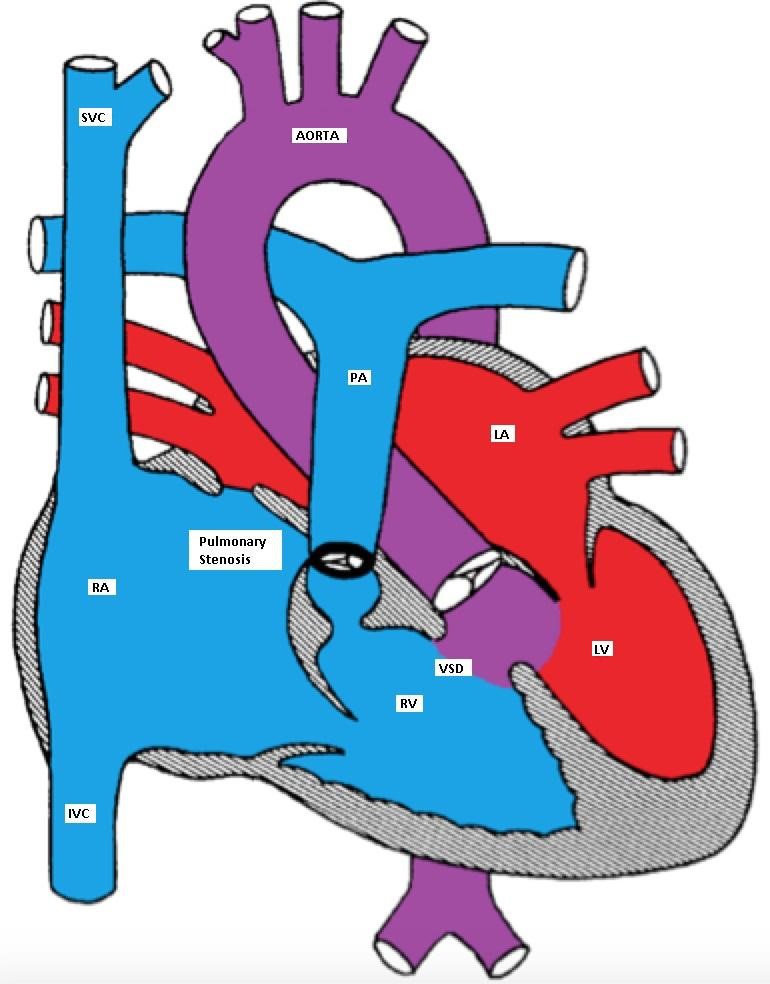
Tetralogy of Fallot (TOF) with large mal-aligned VSD, overriding aorta,
right ventricular outflow tract obstruction and right ventricular hypertrophy
Pathophysiology
The VSD is nonrestrictive (both ventricles have equal pressures). The direction of the blood flow across the VSD depends on the relative systemic and right ventricular outflow tract (RVOT) resistances.
Clinical manifestations
TOF patients usually present with a heart murmur at birth with variable degree of cyanosis (depending on the degree of right ventricular outflow tract obstruction (RVOTO)). Many patients are completely acyanotic (pink TOF). There is a right ventricular tap at the left sternal border, systolic ejection murmur due to RVOTO, and single S2. The VSD is usually non-restrictive and does not produce a heart murmur. Clubbing of digits may be seen in untreated TOF with prolonged cyanosis.
Making the Diagnosis
- EKG: RAD, RVH
- Echocardiography is diagnostic; 25% of patients have right aortic arch and 5% have coronary artery anomalies
- CXR: 'Boot shaped' heart due to concave main pulmonary artery segment and upturned RV apex due to RVH
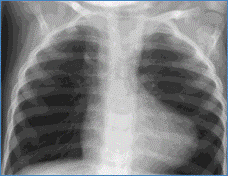
Chest X-ray showing boot shaped heart in Tetralogy of Fallot
Tet spells (hypercyanotic or hypoxic spells):
Tet spells are characterized by paroxysmal cyanosis, tachycardia, tachypnea and irritability. These spells are caused by reversal of the shunt across the VSD so the blood flows from the right to the left ventricle due to an increase in the RVOTO resistance. Initially, the systolic murmur gets louder and then diminishes as the RVOTO progresses to near complete obstruction. Tet spells are more common in the warm weather and in the morning hours when the systemic vascular resistance is low due to increased parasympathetic tone and decreased intravascular volume; a warm bath can have a similar effect. The RVOTO is worsened by increased catecholamine secretion due to irritability, hunger, or wet diapers.
Hypercyanotic spells need urgent treatment; if left untreated, metabolic acidosis, seizures, cerebrovascular accidents or even death may occur. The concept of treatment is to reverse the mechanism of the hypoxic spells. It is important to remove any source of irritation for the infant to decrease the catecholamine surge and worsening of the RVOTO.
While the mother is comforting the baby (nursing, bottle feeding), the baby is placed in a knee-to-chest position to increase systemic vascular resistance. Blow-by O2 is provided to enhance the oxygenation of blood and increase the systemic vascular resistance. If the spell continues, morphine sulphate (0.2 mg/kg) may be given intramuscularly to calm the patient and relax the obstruction of the RVOT. An IV line should be inserted and isotonic fluid boluses should be administered (and may be repeated) until the heart rate normalizes.
Acidosis should be treated with sodium bicarbonate. If the spell continues, vasoconstrictors such as phenylephrine may be tried. Intravenous beta blockers (propranolol or esmolol) may be used to decrease the cardiac contractility and, hence the RVOTO. Beta blockers also slow down the heart rate and therefore, enhance the coronary perfusion. If the spell continues despite all these measures, endotracheal intubation and general anesthesia are indicated in preparation for urgent surgical intervention.
Management
If the patient is pink and has never had a cyanotic spell, elective surgery is usually performed in the first few months of life by closing the VSD and relieving the RVOTO. If the patient is cyanotic or develops a cyanotic spell, intervention is necessary in the neonatal period. If neonatal repair cannot be done because of prematurity or an unstable medical condition, then a palliative shunt between the aorta or one of it's branches and the pulmonary artery is placed to bypass the RVOTO. The classic shunt is called Blalock-Taussig (BT) shunt.
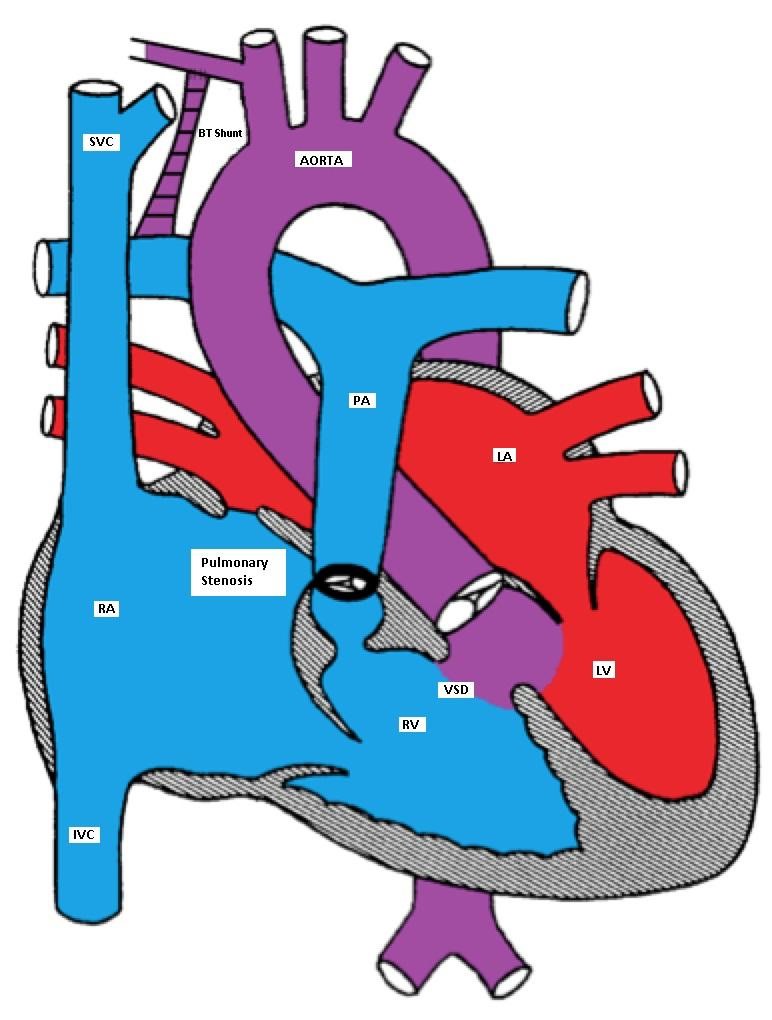
Tetralogy of Fallot (TOF) with a BT shunt between the right subclavian and the right pulmonary arteries
Total Anomalous Pulmonary Venous Connection (TAPVC)
TAPVC accounts for about 1% of all CHD. It is also referred to as total anomalous pulmonary venous return (TAPVR), which is less accurate as the connection may be obstructed and there may be diminished or no pulmonary venous return.
In TAPVC, the pulmonary veins connect to the right atrium or great veins (SVC or IVC) instead of connecting to the left atrium. An atrial communication is necessary to maintain life.
Pathophysiology
In the right atrium, both oxygenated and deoxygenated blood completely mix, and the mixture is then distributed to other cardiac chambers. The oxygen saturation is characteristically the same in all cardiac chambers. There are four different types of TAPVC:
- Supracardiac: The pulmonary veins connect via a vertical vein to the innominate vein and the SVC.
- Cardiac: The pulmonary veins connect to coronary sinus or the right atrium.
- Infracardiac: A common pulmonary vein crosses the diaphragm and connects to the portal vein, ductus venosus, hepatic vein or the IVC.
- Mixed type is a combination of the above.
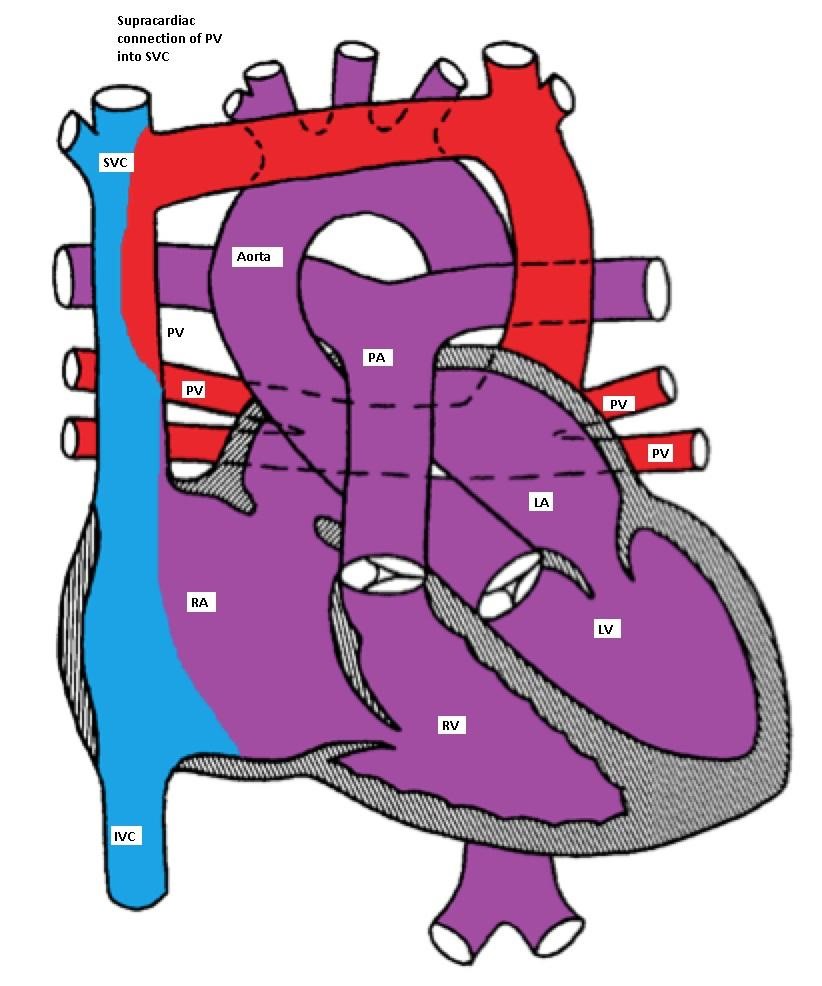
Supracardiac type TAPVC with drainage of pulmonary veins into superior vena cava (SVC)
via vertical vein, which connects to the innominate vein and finally drains into SVC
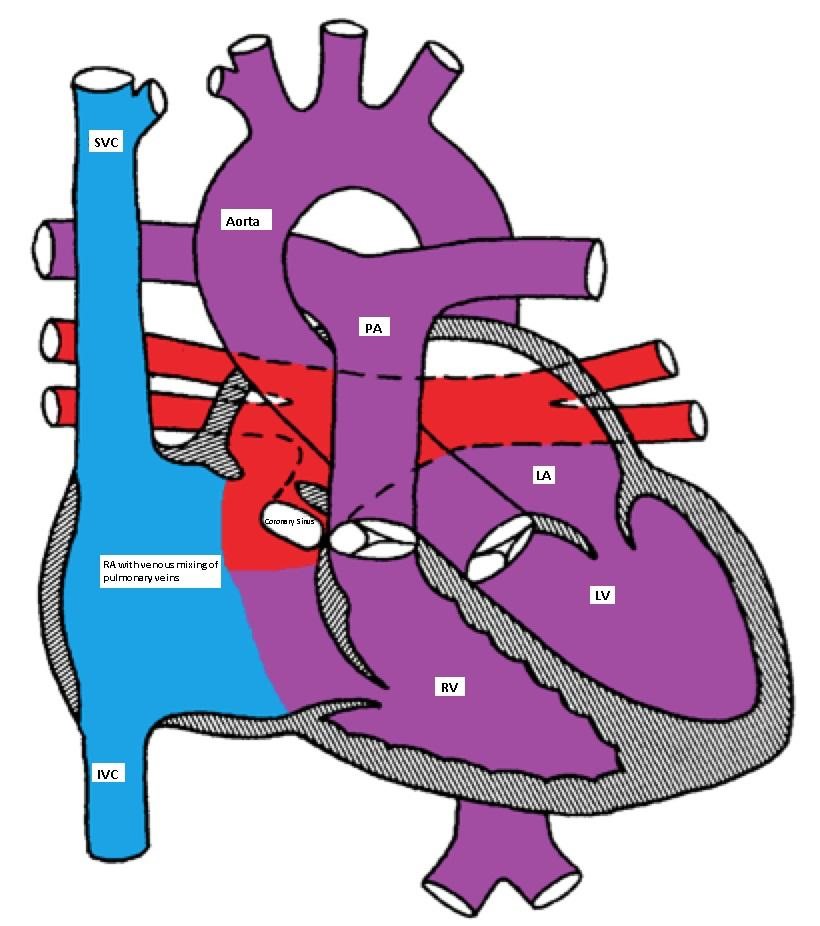
Cardiac type of TAPVC with drainage of pulmonary veins directly into right atrium via coronary sinus
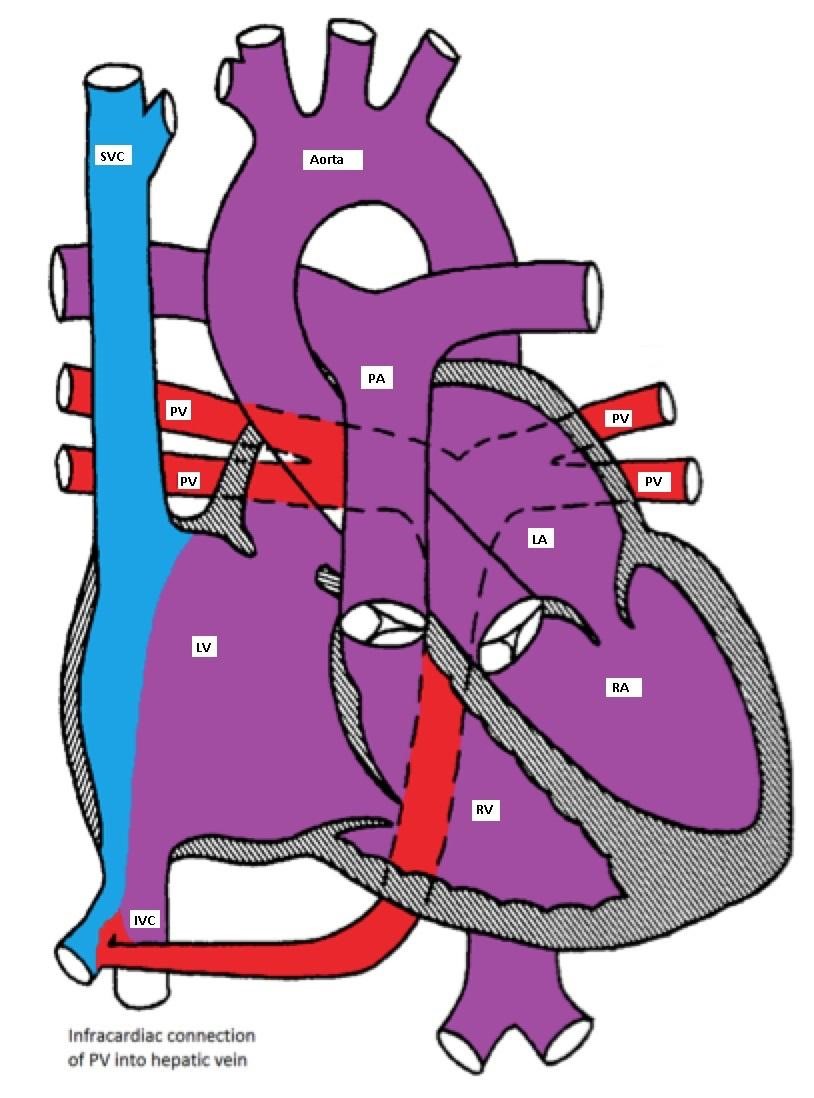
Infracardiac type TAPVC with drainage of pulmonary veins into inferior vena cava via hepatic vein
Clinical manifestations
The clinical manifestations depend on the presence or absence of obstruction to the pulmonary venous connection. If there is no obstruction, mild cyanosis, CHF and frequent pulmonary infection are the common manifestations. With obstruction, there is marked cyanosis, respiratory distress and pulmonary congestion.
Making the Diagnosis
- CXR shows pulmonary edema.
- Cardiomegaly with a "snowman" sign may be seen in supracardiac type.
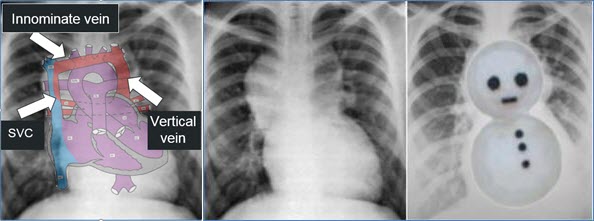
Chest X-ray showing snowman sign of supracardiac TAPVC
Management
Medical management may be tried in patients with TAPVC without obstruction in the form of diuretics and correction of metabolic acidosis. PGE1 causes pulmonary vasodilatation and should be avoided as it may worsen the CHF. All patients with TAPVC will need surgical repair as soon as the diagnosis is made; this includes having the pulmonary veins re-implanted in the left atrium.
Tricuspid Atresia (TA)
TA is a rare form of CHD (1% of all CHD). The RV is hypoplastic and an ASD is necessary for survival. If the ventricular septum is intact, the pulmonary valve would also be atretic. If a VSD is present, there will be a variable degree of hypoplasia of the pulmonary valve depending on the size of the VSD.
TA is classified according to the presence or absence of VSD or TGA. In one fourth of the patients, there is a TGA. Patients with TA with normally related great vessels may be more cyanotic than those with TGA as the degree of cyanosis is inversely related to the volume of the pulmonary blood flow (Figures).
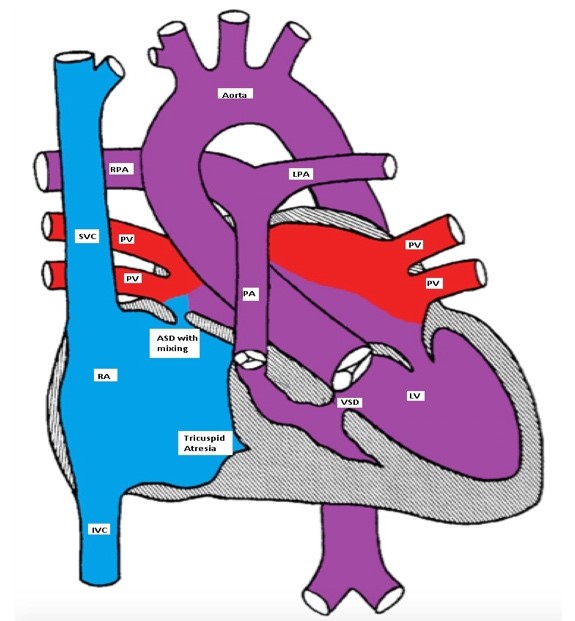
Tricuspid atresia with a restrictive VSD, pulmonary hypoplasia and normally related great arteries
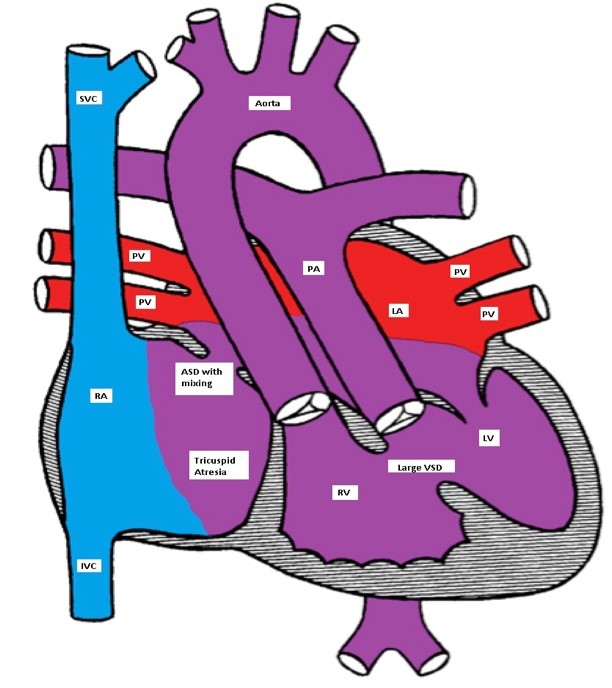
Ticuspid atresia with a non-restrictive VSD and D-TGA
Clinical Presentation, EKG and, imaging
Patients with TA are cyanotic and have a single S2. A holosystolic murmur of VSD may be heard. A PDA murmur may also be heard.
EKG classically shows a left superior axis deviation (due to posterior and inferior displacement of AV node) and diminished RV forces. LVH may be present. Echo is usually diagnostic.
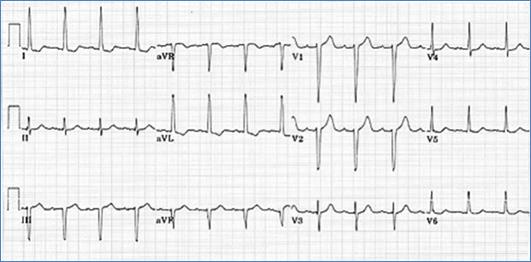
Left superior axis deviation and reduced right ventricular forces consistent with tricuspid atresia
Management
Management includes IV infusion of PGE-1 to maintain the ductal patency. CHF needs to be treated if present. Rashkind atrial septostomy may be needed if the ASD is restrictive. Patients with TGA and hypoplastic RV will need multi-stage single ventricle repair (see Fontan-type operation in HLHS).
Pulmonary Atresia with Intact Ventricular Septum (PA-IVS)
PA-IVS occurs in 2.5 % of patients with CHD.
There is no direct communication between the RV and the main pulmonary artery (MPA) as the pulmonary valve is atretic. The RV is usually hypoplastic. An atrial communication is needed for survival. The pulmonary blood flow is dependent on the patency of the ductus arteriosus.
Clinical Presentation, EKG, imaging
Clinically, patients are severely cyanotic and have respiratory distress. The S2 is single and a PDA murmur may be heard. EKG shows RAE, LVH and possibly RVH.
CXR usually shows reduced pulmonary vasculature.
Echo is diagnostic.
Management
- PGE-1 should be started to maintain the ductal patency.
- Cardiac catheterization is usually needed for the evaluation of the type of PA (membranous vs. muscular) and to detect the presence of coronary sinusoids.
- Balloon atrial septostomy may be performed in the cardiac cath lab.
- Perforation of a membranous atretic pulmonary valve (by radiofrequency) may be attempted.
- If the RV size is adequate, trans-annular patching across the pulmonary outflow may be done.
- In patients with RV dependent coronary circulation, RV decompression may lead to reversal of the coronary blood flow to the RV producing myocardial ischemia. In this case, a BT shunt is placed in preparation for a single ventricle repair.
Ebstein Anomaly of the Tricuspid Valve (EA)
Ebstein anomaly is a rare form of CHD (1%).
There is a downward displacement of the septal and posterior leaflets of the tricuspid valve into the RV cavity creating a large atrialized portion of the RV. The functional RV may be hypoplastic, dilated and thin-walled. There is usually an inter-atrial communication such as PFO or ASD. Ebstein anomaly may be associated with other CHD such as PS, TOF, pulmonary atresia or VSD.
Dysrhythmias, especially Wolff-Parkinson-White (WPW) and SVT are common.
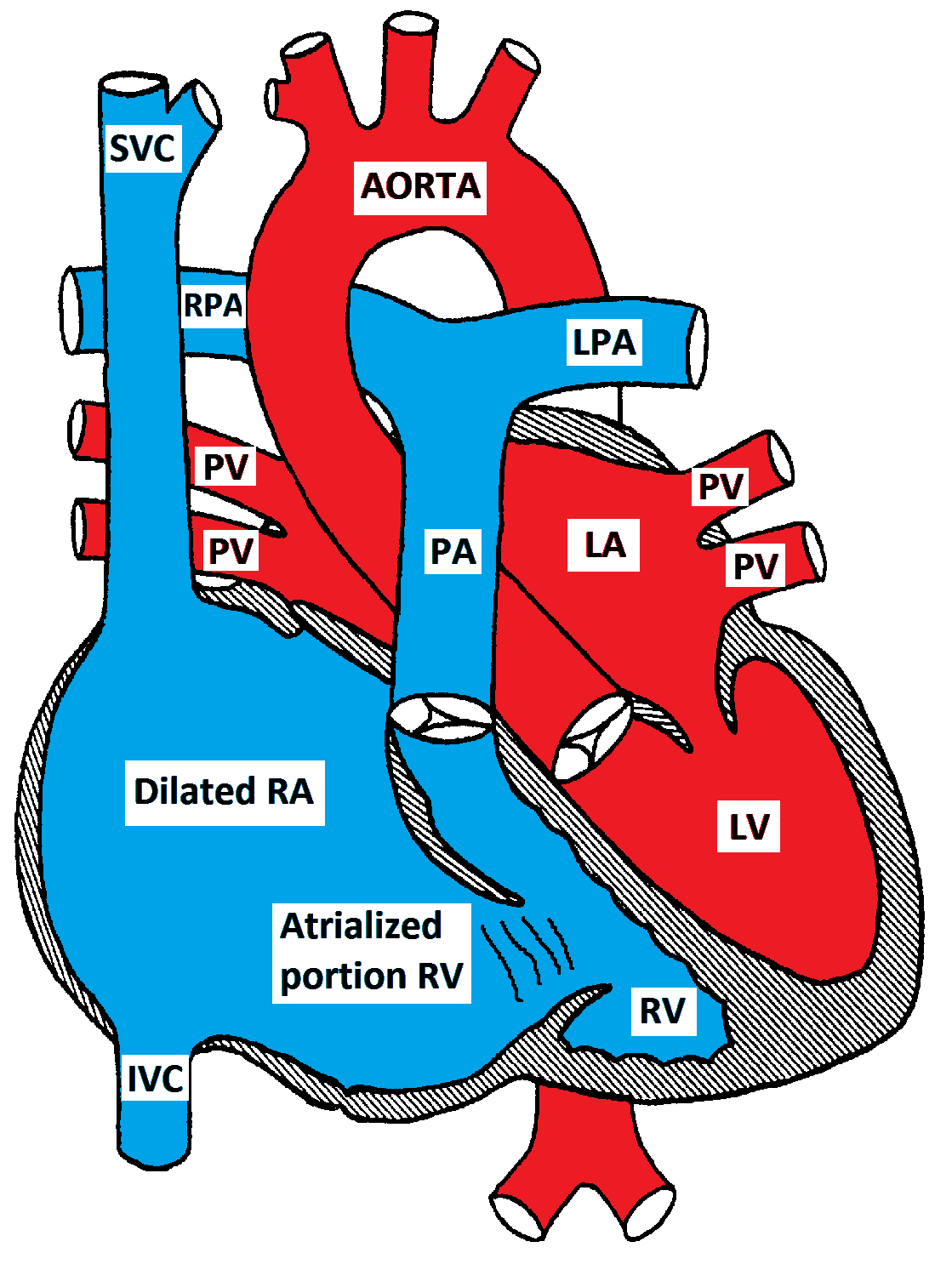
Ebstein Anomaly of the tricuspid valve
Clinical Presentations, EKG, imaging
Infants may present with cyanosis which usually improves as the pulmonary vascular resistance drops. Older children usually present with dyspnea, fatigue and palpitations (SVT). A quadruple rhythm may be audible in addition to a holosystolic murmur at the left lower sternal border due to tricuspid insufficiency. A mid-diastolic murmur due to relative tricuspid stenosis may be heard at the same location. EKG is characterized by massive right atrial enlargement (RAE), RBBB and sometimes first degree AV block or WPW. CXR shows cardiomegaly due to RAE. Echocardiogram is diagnostic. The degree of TR and the size of the RV should be determined.
Management
Medical management is recommended in mild forms. This includes management of CHF and treatment of dysrhythmias. Oxygen supplementation is helpful early in life as it decreases the pulmonary vascular resistance. PGE-1 may be needed to maintain the ductal patency in severe cases. Surgery is required for severe forms and involves reconstruction or replacement of the tricuspid valve and closure of the ASD. If the RV is severely hypoplastic, a single ventricle repair (Fontan procedure) may be necessary.
Hypoplastic Left Heart Syndrome (HLHS)
Hypoplastic Left Heart Syndrome (HLHS) occurs in about 1% of CHD and involves hypoplasia or atresia of the left sided structures including the LA, mitral valve, LV, aortic valve and ascending aorta. A shunt at the atrial level (ASD or PFO) is necessary for survival. A PDA is essential for providing blood supply in a retrograde direction to the transverse and ascending aorta to provide blood to the neck vessels and coronary arteries.
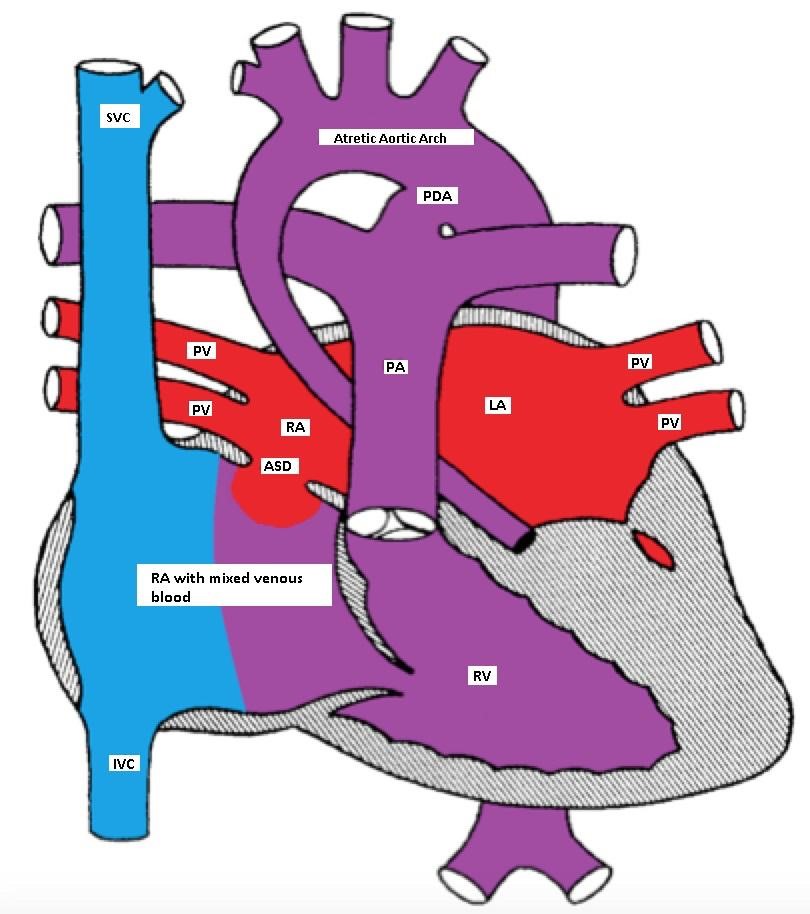
Hypoplastic left heart with a non-restrictive ASD and a PDA
Pathophysiology
There is an obligatory L to R shunt at the atrial level. Both pulmonary and systemic venous blood completely mix in the RA. The RV pumps blood to both systemic and pulmonary circulations. Initially, both resistances are nearly equal and the blood is distributed equally to the pulmonary and systemic circulations. As the pulmonary vascular resistance drops in the first few days of life, more blood flows to the lungs causing pulmonary congestion, reduced systemic flow causing tissue hypoxemia, metabolic acidosis, shock, or even death.
Clinical Presentations
Neonates with HLHS usually present with one of three clinical scenarios:
- Respiratory distress due to pulmonary congestion (CHF) as the pulmonary vascular resistance drops in the first few days of life
- Shock as the ductus arteriosus constricts or
- Cyanosis which is usually mild due to excessive pulmonary flow. Severe cyanosis is uncommon and may indicate inadequate inter-atrial communication or persistent pulmonary hypertension.
A systolic ejection murmur may be heard at the ULSB due to increased flow through the pulmonary valve. A holosystolic murmur due to tricuspid insufficiency may be heard at the LLSB. S2 is usually loud and single. The peripheral pulses may be weak and the skin may be mottled due to poor tissue perfusion.
Making the Diagnosis
- This condition can be diagnosed prenatally as early as 16 weeks of gestation.
- EKG: EKG findings are nonspecific and may show poor progression of LV forces and myocardial ischemic changes.
- Echocardiography is diagnostic and also helps to determine the size of the inter-atrial communication, the patency of the ductus arteriosus, RV function and the presence of tricuspid insufficiency.
- CXR shows cardiomegaly, pulmonary venous congestion and pulmonary edema.
- Cardiac catheterization is rarely necessary for diagnosis but may urgently be needed to perform balloon atrial septostomy if the ASD is restrictive.
Management
Medical management:
- PGE-1 should be started to maintain the ductal patency.
- Avoid oxygen supplementation because it causes pulmonary vasodilatation and lowers the PVR, which makes pulmonary congestion and CHF worse. This may also compromise the systemic blood flow as oxygen is a systemic vasoconstrictor.
- Avoid administration of excessive IV fluids as most of the fluid will go to the lungs, worsening the CHF symptoms.
- Also avoid high doses of inotropic agents as this causes systemic vasoconstriction and increases the SVR. This may accentuate tissue hypo-perfusion and metabolic acidosis. It also increases the myocardial oxygen demand by increasing the contractility.
Surgical management: both multi-stage palliative surgery and heart transplantation have comparable outcomes.
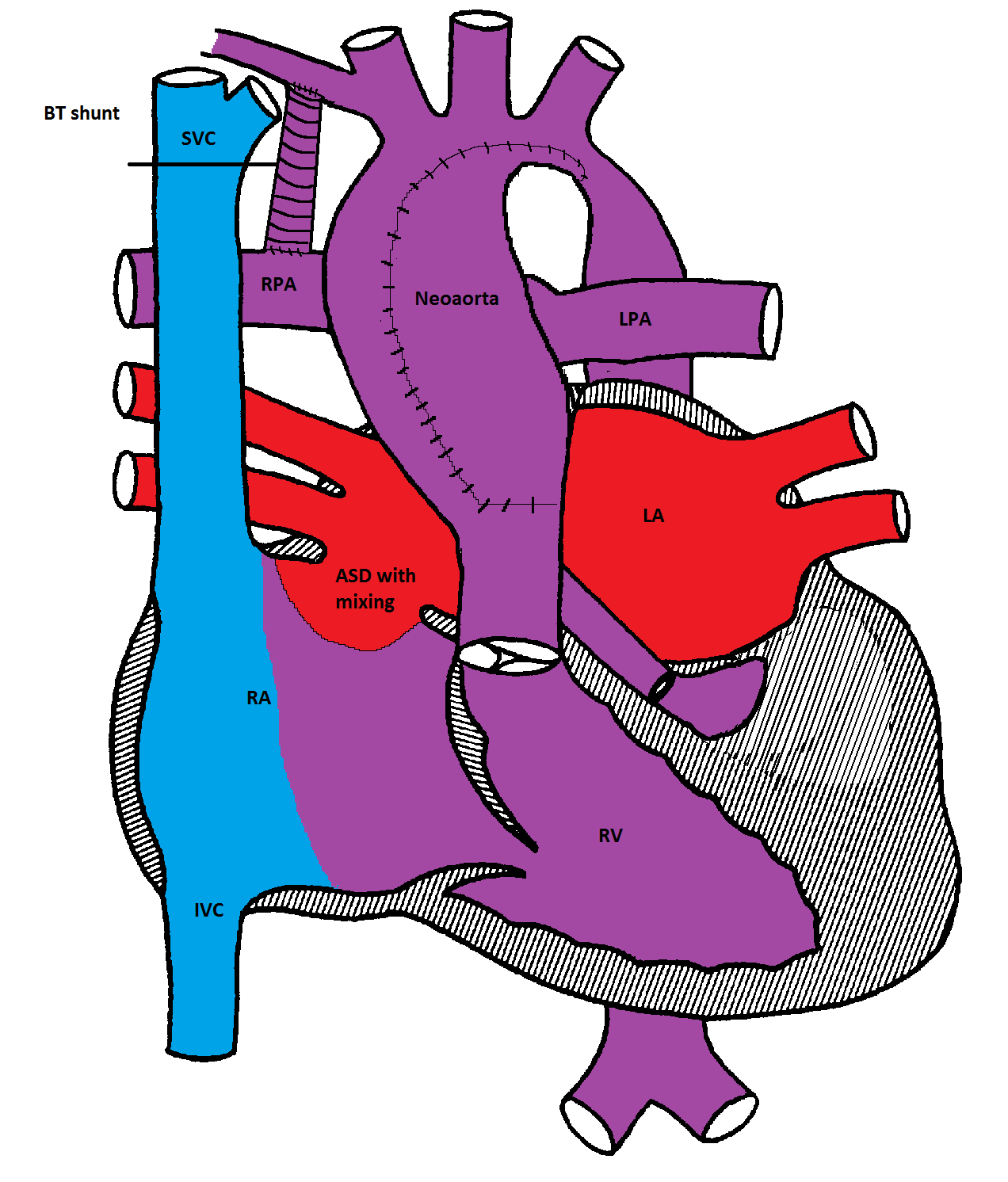
Norwood procedure
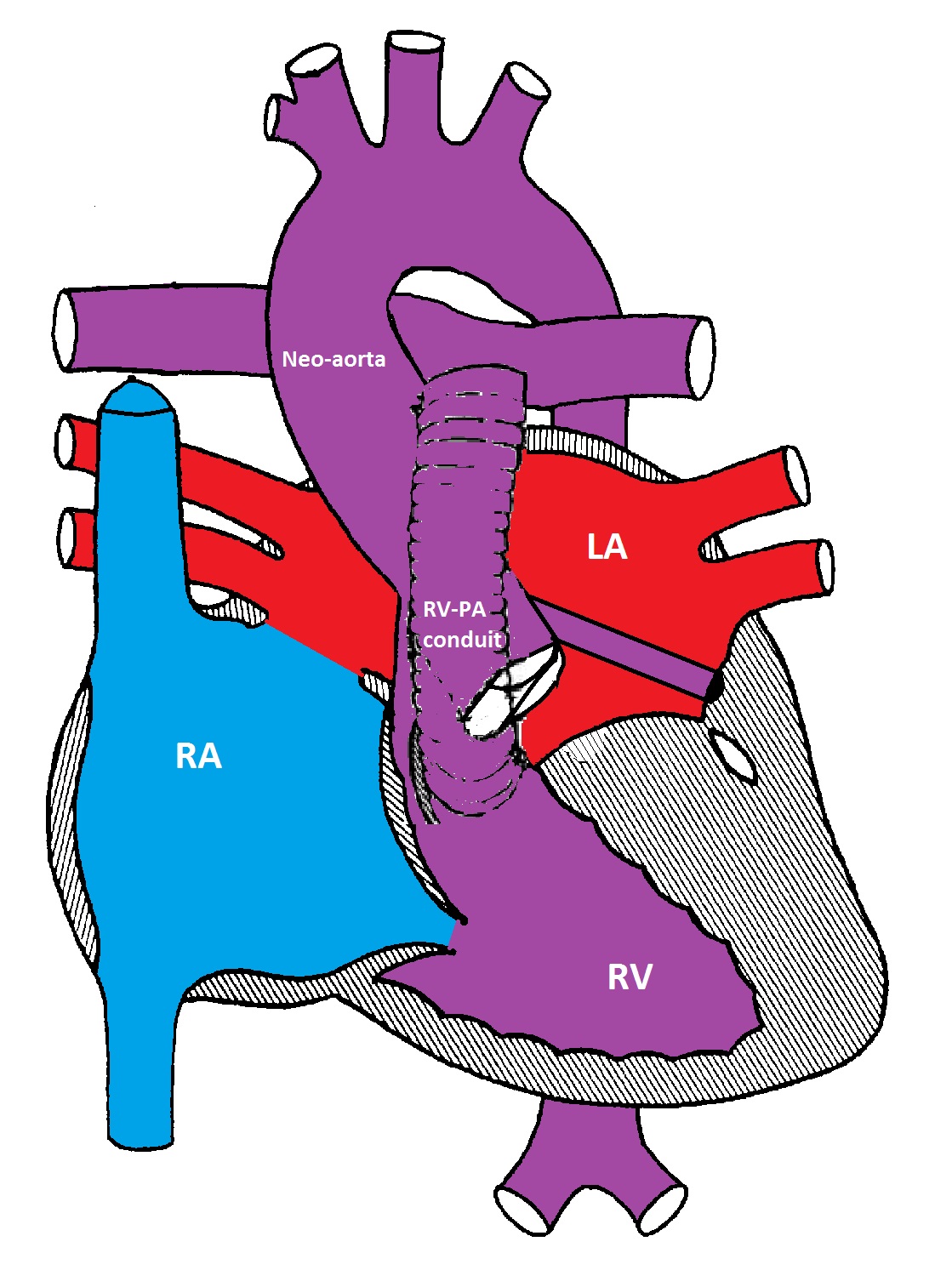
Modified Norwood procedure with a Sano shunt between the right ventricle and the pulmonary artery bifurcation
The first stage (Norwood procedure) is performed in the neonatal period and involves division of the MPA and ligation of the ductus arteriosus, removal of the atrial septum and reconstruction of a new ascending aorta using the proximal MPA and the ascending aorta. A BT shunt made from Gore-Tex® is placed between the innominate artery and the right pulmonary artery to supply blood to both lungs. Because there is complete mixing of blood in the RV, the oxygen saturation is usually in the low 80's%.
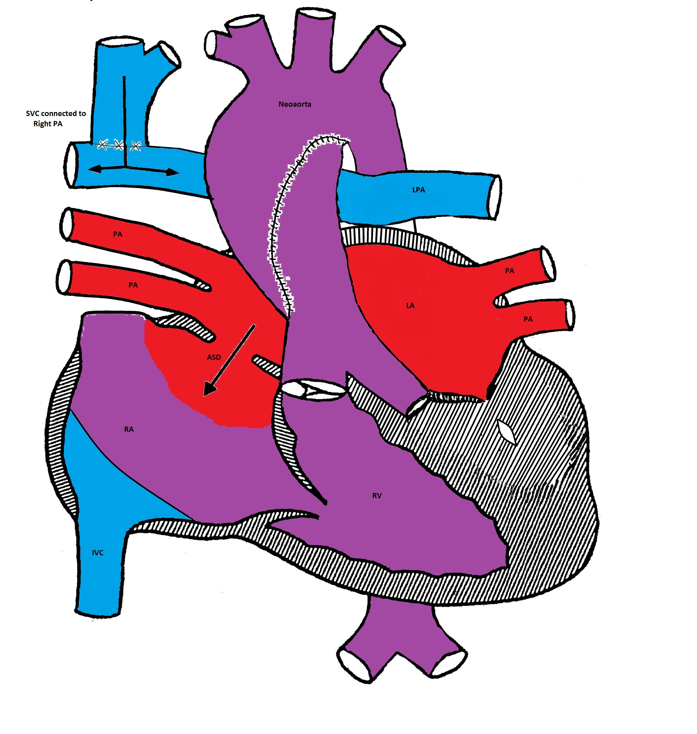
Bidirectional Glenn procedure
The second stage is called bi-directional Glenn or hemi-Fontan procedure and is usually performed at 6 months of age. The SVC is connected to the right pulmonary artery and the BT shunt is removed. The systemic venous blood from the SVC is the only source of pulmonary circulation. The IVC venous blood mixes with the pulmonary venous blood in the RV. The systemic saturation is usually in the mid to high 80's%.
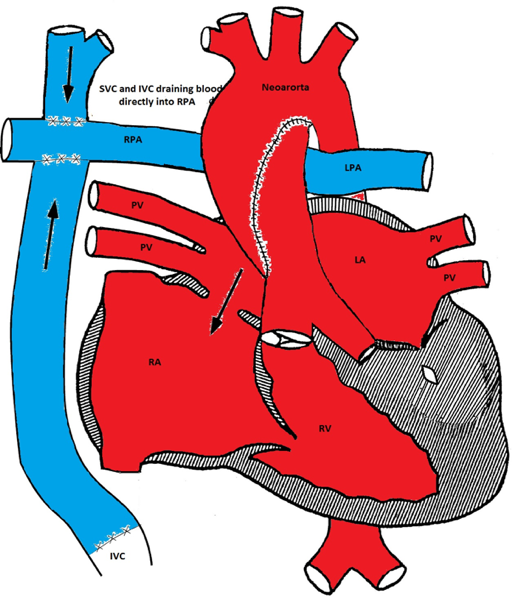
Modified Fontan procedure
The third stage is the Fontan procedure which is usually performed at 18-24 months of age. It involves connecting the IVC to the right pulmonary artery. After this procedure, both systemic and pulmonary circulations are separated. The systemic venous blood from the SVC and IVC passively flows to both lungs via the pulmonary arteries. The RV acts as the systemic ventricle. The oxygen saturation is usually in the low to mid 90% (as there is still some mixing from the coronary venous blood via the coronary sinus).
The Fontan procedure has many complications including an increase in systemic venous pressure, development of chylothorax, and diaphragmatic and vocal cord paralysis. Other complications include atrial tachy-arrhythmias, sick sinus syndrome, and protein-losing enteropathy.
Quick Check
