CONGENITALLY ACQUIRED INFECTIONS
This chapter is intended only as a quick reference for the infections encountered most frequently in the nurseries. They are listed here in alphabetical order. Further reading is recommended when caring for a baby with any of these diseases, and consultation with the Pediatric Infectious Disease service may be required.
Cytomegalovirus
Human CMV is a DNA virus, and a member of the herpes virus group. Approximately 1% of all live-born infants are infected in utero and excrete CMV at birth, making this the most common congenital viral infection.
Clinical findings. Congenital CMV infections have a spectrum of manifestations. Usually infection is asymptomatic, but some congenitally infected infants who appear to be asymptomatic at birth are found in childhood to have hearing loss or learning disability.
Approximately 10% of infants with congenital CMV infection are symptomatic at birth. Infants may present with intrauterine growth retardation, neonatal jaundice, purpura, hepatosplenomegaly, microcephaly, brain damage, intracerebral calcifications, and chorioretinitis. Developmental delays are commonly found in later life. Sensorineural hearing loss (SNHL) is the most common sequela of congenital CMV, and it is more common in babies who have additional symptoms. CMV is the leading non genetic cause of SNHL in the US; 21% of all congenital hearing loss (only half are symptomatic) and 25% of all hearing loss at age 4 years is attributable to congenital CMV.
33-50% of hearing loss from CMV occurs after infancy. In pre term infants, transfusion of CMV positive blood products has been associated with lower respiratory tract disease.
Vertical transmission of CMV to the infant occurs (1) in utero by transplacental passage of maternal blood-borne virus, (2) at birth by passage through an infected maternal genital tract, or (3) postnatally by ingestion of CMV-positive human milk. Infection acquired from maternal cervical secretions or human milk is usually not associated with clinical illness.
Severe sequelae are most likely from primary infection in the first half of gestation; however, in utero fetal infection can occur regardless of whether the mother had primary infection or reactivation during pregnancy.
Infants infected during maternal reactivation are much less frequently affected than those infected due to maternal primary infection, presumably because of immunity in the mother.
Diagnosis. In the asymptomatic baby, congenital CMV is usually diagnosed by isolation from the urine using polymerase chain reaction. CMV may be isolated from CSF in babies with CNS disease.
Treatment. In neonates with symptomatic congenital CMV disease of the CNS, ganciclovir, administered at 6 mg/kg/dose every 12 hours IV for 6 weeks, may protect against hearing deterioration and developmental impairment at 1-2 years. Pre term infants with pneumonitis, hepatitis or thrombocytopenia due to perinatally acquired CMV may be treated with ganciclovir for 2 weeks, assessed for improvement, and treated for an additional 1-2 weeks if deemed beneficial.
Control Measures. Neonates known to be CMV shedders excrete virus in urine and respiratory secretions. Particular attention to good handwashing techniques and the use of gloves when handling contaminated objects such as diapers is important, particularly in the ICU setting.
Gonorrhea
The etiologic agent of gonorrhea is Neisseria gonorrhoeae, a gram negative, oxidase positive diplococcus. Gonorrhea is most often an asymptomatic infection during pregnancy, but it may be associated with an increased risk of perinatal morbidity and mortality including fetal wastage, early and prolonged rupture of the membranes, and delivery of low birth weight babies. Intrapartum infection may result in disseminated disease with bacteremia, arthritis and meningitis, or scalp abscess if intrauterine fetal monitoring is done. Genital gonococcal (GC) infection is often associated with neonatal gonococcal ophthalmia.
Diagnosis of GC infection. The organism can be cultured or identified by nucleic acid amplification, using techniques such as PCR and transcription-mediated amplification.
Treatment of the Newborn.
Prophylactic Measures. Ophthalmic prophylactic treatment is administered routinely. Either 1% tetracycline ointment, or 0.5% erythromycin ointment (used at UTMB and most institutions in the US) is instilled into the eyes of every newborn during the transition period. Silver nitrate is no longer recommended for eye prophylaxis.
Asymptomatic babies born to mothers with recognized GC infection receive a single injection (IM or IV) of Ceftriaxone (25-50 mg/kg, not to exceed a total of 125mg). Topical prophylaxis alone is not adequate for these neonates, although GC ophthalmia rarely develops if prophylaxis is administered correctly.
Neonatal Ophthalmic or Disseminated Infections with GC.
- Keep in the hospital; obtain cultures of blood, cerebrospinal fluid and eye discharge.
- For disseminated infections, therapy is Ceftriaxone 25-50 mg/kg/d I.V. or I.M. once daily or Cefotaxime 25 mg/kg/d (recommended for prematures with hyperbilirubinemia) I.V. or I.M. in 2-3 divided doses. Duration of therapy is 7 days unless meningitis is present.
For meningitis, therapy should be extended to 10-14 days. If the organism is sensitive to penicillin, treat with IV aqueous crystalline penicillin G at doses of 100,000 units/kg/day divided q 12 h, for 7-14 days
- Consult ophthalmology
- For ophthalmia only, treat with a single dose of Ceftriaxone. Eyes should be irrigated with saline at frequent intervals to remove purulent exudate. Topical antibiotics are not necessary.
- Babies should be isolated for 24 hours after beginning antibiotics.
- Look for C. trachomatis, congenital syphilis and HIV.
Hepatitis Infections
Hepatitis A (HAV). HAV infection is unusual in pregnancy and there is no chronic carrier state. Viremia and fecal shedding are short‑lived and usually absent or almost gone by the time symptoms appear, so infants born to mothers who have had acute disease are at minimal risk. Specific tests for antibody to hepatitis A (anti‑HAV) are available. IgM‑specific anti‑HAV signifies current or recent infection but may also occur during convalescence. The presence of IgG-specific anti-HAV signifies current or previous infection and indicates immunity.
Hepatitis A is not a neonatal problem unless the mother's symptoms begin between 2 weeks before and one week after delivery. In such cases a single injection of standard gammaglobulin, 0.02 ml/kg IM is recommended shortly after birth.
Hepatitis B (HBV). HBV infection occurs in 80‑90% of infants exposed to HbsAg/HbeAg positive mothers in the 3rd trimester or early postpartum period, and is detectable by 1‑4 months of age. Most infections are asymptomatic; rarely fulminant hepatitis occurs. About 90% of infected babies become chronic HBV carriers, with progression to chronic active hepatitis, cirrhosis, or hepatocellular carcinoma in 25%.
Below is a description of the hepatitis antigens and antibodies as they relate to infection:
- HBSAg, the surface antigen, appears 1‑3 weeks after exposure, and lasts 1‑3 months.
- Anti‑HBs is detected 2 weeks to months after HBsAg disappears, and lasts for many years.This is the antibody which is detectable after successful immunization for HBV.
- Anti‑HBc appears at the onset of the disease and lasts indefinitely.
- HBeAg appears and disappears with HBsAg.
Blood which is positive for HBsAg and HBeAg is highly infectious. Chronic infection is characterized by persistence of raised transaminase levels, HBsAg, HBeAg, and anti‑HBc. Most infants who are infected at birth become HBsAg positive within 4 months, but are usually negative at birth. This indicates that infection usually occurs around the time of delivery ‑ from swallowing amniotic fluid or maternal blood, leakage of virus across the placenta, or through minor abrasions at delivery. Babies born by c‑section are infected as readily as those born vaginally.
Management of HBsAg‑Positive Mothers and Their Infants. The neonate should be bathed carefully as soon as possible after birth to remove maternal blood and secretions. After bathing, the newborn does not require isolation or segregation from the rest of the babies.
- Hepatitis B Immune Globulin (HBIG) (0.5 ml) should be administered intramuscularly (IM) as soon as possible- immediately after physiologic stabilization of the newborn and preferably within 12 hours of birth. Even if a mother is discovered to be HBsAg positive after delivery, administer HBIG; efficacy decreases markedly if treatment is delayed beyond 48 hours, but can be given up to 7 days of age.
- HB vaccine should be administered IM in three doses of 0.5 ml of vaccine (5mcg) each. The first dose should be given within 7 days of birth and may be given concurrently with HBIG but at a separate site. The second and third doses should be given as shown in the chart below.
- HBsAg testing at 6 months may be done for counseling purposes. HBsAg positivity at 6 months indicates a therapeutic failure, and the third vaccine dose need not be given.
- Testing for HBsAg and anti HBs is recommended at 9 months to monitor the final success or failure of therapy. If HBsAg is found, it is likely the child is a chronic carrier. IF HBsAg is not detectable, and anti‑HBs is present, the child has been protected. Since maternal antibody to the core antigen (anti‑HBc) may persist for more than 1 year, testing for anti‑HBc may be difficult to interpret during this period.
- HB vaccine is an inactivated product, and it is presumed that it will not interfere with other simultaneously administered childhood vaccines. HBIG administered at birth should not interfere with oral polio and diphtheria‑tetanus‑pertussis vaccines administered at about 2 months of age.
Breastfeeding is permitted if the infant is appropriately immunized.
The concurrent use of HBIG and HB vaccine has an efficacy of about 90% in preventing chronic infection. The efficacy of HB vaccine alone, given at birth, and repeated at 3 and 6 months is about 75%. Preterm infants weighing < 2 kg whose mothers are HBSAg unknown or positive should receive HBlG and HBV at birth. They should start over routine 3-shot immunization at 2 kg.
HBV prophylaxis for the term infant whose mother is HepBSAg positive is summarized on the following table (see the "Red Book", for alternative scheduling):
|
AGE in mos. HBV Schedule HBV Marker Screening HB Vaccine** 1 mo HB Vaccine 6 mos. HB Vaccine HBsAg test *** 9 mos. HBsAg+ and anti‑HBs tests ++ *HBIG 0.5 ml IM within 12 hrs of birth **HB vaccine 0.5 ml IM within 7 days of birth and at 1 and 6 months ***Optional; if positive, indicates infection, and the third HB vaccine need not be given +If positive, indicates a therapeutic failure ++Anti‑HBs positive indicates a therapeutic success |
Hepatitis C (HCV). HCV is a small single-stranded RNA virus, a member of the Flavivirus family.
The prevalence of HCV infection in the US is estimated at 1.3%. Much like hepatitis A or B, hepatitis C is characterized by mild or asymptomatic infection with jaundice and malaise. Persistent infection occurs in 50-60% of infected children, and they are less likely to develop chronic hepatitis (70-80%) and cirrhosis than adults. HCV is the leading indication for liver transplantation for adults in the US. Vertical transmission is 5-6% and occurs only if the mother is positive to HCV RNA at the time of delivery; if the mother is also HIV positive, the risk of HCV transmission is higher. Breastfeeding does not increase the risk of HCV transmission.
At UTMB, the Pediatric Infectious Disease group has a protocol for follow-up of babies whose mothers have Hepatitis C.
- All newborns of mothers with hepatitis C infection will be identified prior to discharge.
- The affected newborns will have a plan for diagnosis of hepatitis C infection in the outpatient clinic. Mothers will be informed at discharge that the baby is at risk for hepatitis C infection (4-7%) and that diagnosis must be made in the outpatient setting.
- If mothers desire to return to UTMB for hepatitis C testing, a referral to the Pediatric Infectious Disease Clinic will be made at two (2) months of age. The preferred test at this visit is hepatitis C PCR. Additional follow-up testing will be scheduled after this visit (antibody test at 12 months; if positive, repeat at 18 months). A negative antibody test is required for final exclusion of diagnosis.
If the mothers cannot return to UTMB for hepatitis C testing, written information must be given to her to review the need for hepatitis C testing with the primary care physician (PCP) of the baby at the very first clinic visit. The PCP will follow the testing protocol of his/her own preference. AAP guideline recommends early PCR at 1-2 months of age if 'desired, and to do hepatitis C antibody test at 18 months of age. Other published opinions are in favor of early PCR as a routine.
Herpes Simplex
Most babies who are infected with herpes simplex virus (HSV) are born to mothers without a history of HSV. In newborn infants, HSV infection can manifest as 1) disseminated infection involving the liver and other organs, including the central nervous system (encephalitis); 2) localized central nervous system disease; or 3) disease localized to the skin, eyes, and mouth (SEM disease). Ocular manifestations include conjunctivitis, keratitis, and chorioretinitis. Of all cases of neonatal HSV, 20% are disseminated, ~33% are CNS, and the rest are SEM disease. Although common in older children, asymptomatic HSV infection occurs rarely, if at all, in neonates.
Typical vesicular skin lesions are helpful diagnostically, but they are only present in 60-75% of babies with disseminated or CNS disease, and in 80-85% of babies with SEM disease. Therefore, even if the pathognomonic skin lesions are absent, the differential diagnosis of respiratory distress, sepsis, and convulsions in newborn infants must include HSV infection. Initial symptoms can occur shortly after birth or as late as 6 weeks after birth. Disseminated and SEM diseases usually occurs during the first 2 weeks of life; disease localized to the central nervous system more often occurs during the second or third week.
Neonatal herpetic infections frequently are severe, with a high mortality rate and significant neurologic and/or ocular impairment of survivors, particularly in the absence of antiviral therapy. Recurrent skin lesions are often noted in surviving infants and are associated with sequelae if they occur frequently in the first 6 months.
The incidence of neonatal HSV disease ranges from 1 per 3,000 to 1 per 20,000 live births. Infants who develop HSV infection are significantly more likely to have been born prematurely and/or to be of low birth weight. 75% of neonatal infections are caused by HSV-2. HSV is most frequently transmitted to an infant during passage through an infected maternal lower genital tract during birth or by an ascending infection, sometimes through apparently intact membranes. Intrauterine infections causing congenital malformations have been implicated in rare cases. Less commonly, babies may be infected postnatally from the mother or father, usually from a non genital infection, or from another infected infant, probably via the hands of personnel attending the infants. Postnatal transmission from personnel with fever blisters to neonates is extremely rare.
The risk of HSV infection in an infant born vaginally to a mother with a primary (first occurrence) genital infection is high (25% to 60%). The risk to an infant born to a mother with recurrent HSV infection at delivery is much lower- around 2%. Distinguishing between primary and recurrent HSV infection in women by history or physical examination may not be possible. Surveys suggest that 0.01% to 0.39% of American women shed HSV at delivery. Either primary or recurrent infection can be present in the mother without symptoms or nonspecific findings (e.g., vaginal discharge, genital pain, or shallow ulcers). Most infants (about 75%) who develop HSV infection are born to women without history or clinical findings suggestive of active infection during pregnancy.
Prevention of Neonatal Infections
Pregnant Women. All pregnant women should be questioned during a prenatal visit about history of HSV infection in themselves or in their sexual partners, and signs and symptoms of current infection should be sought as part of prenatal care.
Women in Labor. During labor all women should be questioned about recent and current HSV symptoms and carefully examined for evidence of genital HSV infection. Cesarean delivery of women in labor who have clinically apparent HSV infection (particularly primary infection) may reduce the risk of neonatal HSV infection unless the membranes have been ruptured for more than 4 hours. The risk in situations where the membranes have been ruptured for longer periods is uncertain, but many obstetricians prefer to deliver infants by cesarean section whenever the birth canal is infected regardless of membrane rupture.
A history of genital HSV for a woman in labor is not an indication for cesarean section. Scalp monitors should be avoided when possible in infants of women suspected of having genital herpes. The use of antiviral therapy in women with a history of HSV is unproven in efficacy and safety.
Infected Hospital Personnel. The risk of transmission to infants by personnel who have oral labial HSV infection ("cold sores") or who are asymptomatic oral shedders of the virus is not known, but probably is low. Personnel with cold sores who have indirect contact with infants should cover the lesions and avoid touching the sores. Of course they should also carefully observe handwashing policies, and must not kiss or nuzzle newborn infants or children with dermatitis. Personnel with herpetic whitlow should not have responsibility for direct care of neonates, immunocompromised patients, or patients in an intensive care unit.
Evaluation for Suspected HSV. Collect the following specimens for culture (best to place in viral transport media):
Symptomatic. Skin vesicles, mouth/nasopharynx/conjunctivae/anus (single swab), blood, and CSF. CSF should also be sent for routine indices and HSV PCR. Hepatic transaminases should be determined.
Asymptomatic. A swab specimen from mouth, nasopharynx, conjunctivae and anus can be obtained with a single swab ending with the anus and placed in one viral transport media tube. Full work-up should be done if the cultures are positive and treatment is initiated.
Care of Exposed Newborns
Infants Born to Mothers with Active Genital Lesions. The infant should be observed carefully for skin or scalp rashes, especially vesicular lesions, and unexplained clinical manifestations such as respiratory distress, seizures and signs of sepsis. If any of these occur, the infant should be evaluated for possible HSV as well as for bacterial infection. Skin lesions and a swab specimen as above should be cultured for HSV. Start Acyclovir if culture(s) from the infant are positive, if HSV infection is strongly suspected while awaiting culture results, if bacterial cultures are negative, or if no other cause of the infant's clinical manifestations is found.
Obtain HSV surface cultures as above for asymptomatic infants at 24 hours of age, since positive HSV cultures obtained at this time are more likely to indicate infection than transient colonization from intrapartum exposure. If surface cultures are positive, the baby should receive a full evaluation as for the symptomatic baby; if work-up is negative, the baby should be treated with acyclovir for 10 days to prevent HSV disease (2012 RedBook).
Infants Born to Mothers With History of Genital HSV but Without Active Lesions at Delivery. These infants should be carefully evaluated for clinical evidence of HSV. It is not necessary to obtain surface cultures in these babies routinely.
- The length of in-hospital observation for infants at increased risk for neonatal HSV is empiric and based on factors specific to the infant's home, availability of follow-up care, and clinical assessment. Two to five days is reasonable.
- Delay elective or ritual circumcision for about a month for infants at highest risk of disease, ie not while in newborn nursery.
- Since neonatal HSV infection can occur as late as 6 weeks after delivery, physicians must be alert for a new rash or symptoms which might be due to HSV.
Treatment of Neonatal HSV infection.
Acyclovir is the treatment of choice for neonatal HSV infection. The dose is 60 mg/kg/d in 3 divided doses, given intravenously for 14-21 days, with the shorter duration used for SEM disease. Relapse of disease after cessation of treatment can occur and is usually related to host factors, but acyclovir resistance has been reported. Retreatment of infants with recurrent skin lesions is advisable due to association with CNS sequelae if occurring in 1st 6 months of life. Prolonged oral administration of acyclovir has been associated with neutropenia.
Infants with ocular involvement due to HSV infection should receive a topical ophthalmic drug (specifically, 1% trifluridine, 0.1% iododeoxyuridine, or 3% vidarabine), as well as parenteral antiviral therapy. Ophthalmologic consultation must be obtained.
All infants surviving neonatal herpes should receive 6 months of oral acyclovir suppressive therapy.
The best outcomes are associated with SEM disease. Most babies with CNS involvement have substantial sequelae, and the mortality of disseminated disease is 20%.
Control Measures and Isolation of the Hospitalized Patient
Neonates With HSV Infection or with positive cultures in the absence of disease, should be hospitalized in a private room, if possible, and managed with contact isolation as long as lesions are present.
Neonates Exposed to HSV During Delivery may be in the incubation phase of infection and should be carefully observed. One method of infection control is to have the infant room-in continuously with the mother in a private room. Infants born vaginally (or by cesarean delivery if membranes have been ruptured for more than 4 hours) to a mother with active HSV lesions should be physically separated from other infants and placed in contact isolation (and in an incubator) if they are hospitalized in the nursery during the incubation period.
Women in Labor and Postpartum Women with HSV Infection should be managed during labor, delivery, and the postpartum period with contact or drainage/secretion precautions, depending on the extent of the mucocutaneous disease. These mothers should be instructed on the importance of careful hand washing before and after caring for their infant. A clean covering gown may be used to help avoid contact of the infant with the lesions or infectious secretions. A mother with herpes labialis ("cold sores") or stomatitis should wear a disposable surgical mask when touching her newborn until the lesions have crusted and dried. She should not kiss or nuzzle her newborn until the lesions have cleared. Herpetic lesions on other skin sites should be covered. Breast feeding is acceptable if no lesions are present on the breast and if active lesions elsewhere on the mother are covered.
HIV/AIDS
HIV type 1 (HIV-1) is an RNA retrovirus which requires the activity of a viral enzyme, reverse transcriptase, to convert the viral RNA to DNA, and is more common in the US than HIV-2.
Approximately 100-200 infants with HIV-1 infection are born annually in the US; mother-to child transmission of HIV-1 accounts for virtually all new infections in preadolescent children. The usual onset of symptoms is 12-18 months of age for untreated, perinatally infected children. HIV-positive mothers should not breast feed nor donate milk because HIV DNA has been detected in both the cellular and cell-free fractions of human breast milk. Transmission of HIV via breast milk has been documented (0.7%per month- this is true even if they have an undetectable viral load.)
Transmission/Incubation. The risk of infection for an infant born to an HIV-seropositive mother who did not receive preventive treatment is between 12% and 40%. Less than half of transmission events occur in utero; most occur during the perinatal period. The risk of transmission is decreased by cesarean section delivery.
Management of Infants Born to Mothers with HIV/AIDS. Standard precautions should be followed. The risk of transmission to healthcare personnel is minimal. The risk of HIV transmission to the newborn is significantly reduced by a maternal-newborn regimen of zidovudine. It is extremely important that a baby born to an HIV-positive mother be started on oral zidovudine (ZDV, or AZT) within 6-12 hours of birth. ZDV can be started before lab tests are drawn. For preterms <35 wks or babies who are NPO, consult with I.D. before beginning ZDV.
|
Dosing |
Duration |
|
|
Zidovudine |
≥35 weeks gestation:
Zidovudine should be started as soon after birth as possible and preferably within 6-12 hours of delivery
< 35 weeks gestation:
-- 1.5 mg/kg/dose IV q8h -- 2 mg/kg/dose PO q8h
-- 1.5 mg/kg/dose IV q8h -- 2 mg/kg/dose PO q8h |
Birth through 6 weeks |
|
2-drug regimen:
and Nevirapine |
Two drug regimen for high-risk neonates only Zidovudine: dose as above
Nevirapine Choose one of the following doses based on weight group, give as three doses, once on day 1, 3 and 7, as in the right side column: Birth weight 1.5-2 kg: 8 mg per dose give orally Birth weight >2 kg: 12 mg per dose given orally |
Zidovudine: Birth through 6 weeks
Nevirapine: 3 doses in the first week of life |
Diagnosis and Follow-up of HIV. See separate practice guideline.
Syphilis
The etiologic agent of syphilis is Treponema pallidum. Pregnant women should be screened with a serological test for syphilis (STS) at the first prenatal visit and then within one week of delivery. Both maternal results should be available before discharge of the infant.
The UTMB laboratory has recently changed to the syphilis IgG as a screening test for syphilis, which is confirmed with another specific treponemal test, the FTA‑ABS (Fluorescent Treponeme Antibody Absorption Test).
The RPR (Rapid Plasma Reagin) is used to determine titers if the treponemal tests are positive. Other hospitals and clinics may use the VDRL (Venereal Disease Research Laboratory). The RPR (or VDRL) tests for the presence of antibodies (IgG subclass) directed against the lipoidal antigen that results from interaction of body tissues with the spirochete when T.pallidum infection occurs. However, other diseases can cause this antigen to appear and stimulate antibody production. Some of these diseases include collagen vascular disorders, mononucleosis, tuberculosis, hepatitis, certain other viral infections as well as drug abuse. Therefore, a mother may have a positive RPR but may not presently have or have ever had syphilis (i.e., she has a biological false positive test.). A mother with a biologic false positive RPR will not have a positive FTA‑ABS, nor will her baby. Because the screening test for syphilis at UTMB is a treponemal test, we rarely see biologic false positive tests, but most centers begin with a non-treponemal test and confirm with a treponemal test.
With acquired syphilis, there may be a period of from 10‑90 days (mean 3 weeks) before the RPR becomes positive. With proper treatment the titer gradually decreases and after about 2 years the RPR usually reverts to negative. In contrast, the treponemal tests become positive early and stay positive for life in most people, despite adequate treatment.
Because the antibodies identified by the RPR and FTA-ABS or IgG tests are IgG antibodies, they readily cross the placenta. Therefore, a baby born to a mother who has active syphilis, treated syphilis, or biological false positive test will most likely have a positive RPR with a quantitative dilution titer about the same as that of the mother. Likewise, the baby of a mother with a positive FTA‑ABS will have a positive FTA‑ABS, so it is not useful to obtain an FTA-ABS on the baby.
The FTA‑ABS and IgG tests are qualitative tests and give results as either positive or negative. The RPR and VDRL are both qualitative and quantitative. The quantitative dilution titers are reported with positive tests. A titer of 1:32 or greater in the baby or a titer >4 times greater than the mother's titer is very suggestive of fetal infection. Note that the titers derived from the RPR or VDRL tests are not interchangeable. A reactive RPR with a titer of 1:16 is not comparable to a reactive VDRL with a 1:16 titer.
CSF serology is done using the VDRL while blood is used to do the RPR. FTA‑ABS is not done on CSF.
If the mother has a positive syphilis IgG, the clinical laboratory follows the algorithm below for further evaluation of the mother.
Neonatal Syphilis. A baby rarely has overt congenital syphilis at birth. The symptoms include snuffles, hepatosplenomegaly, rash, bullous eruption on palms and soles, lymphadenopathy, anemia, thrombocytopenia, conjugated hyperbilirubinemia, pneumonia, bone changes, fetal hydrops, and nephrosis/nephritis. More often babies return to the hospital at 2 to 3 weeks of age with these symptoms after having been sent home from the nursery where they were asymptomatic.
Evaluation of the ASYMPTOMATIC newborn with suspected syphilis (i.e., mother has a positive syphilis IgG assay and has not been treated) MUST include RPR on the baby's serum (titer of > 1:32 or > 4 times the mother's titer strongly suggests fetal infection), and a thorough maternal history which includes the dates of any previous serologic tests for syphilis, and the dates of any previous treatment.
The results of the baby's serum RPR and a thorough maternal history can be used to make further decisions.
The evaluation of the baby (and positive results of each study in parentheses) may include
1. Long bone x-rays (metaphysitis, trophic changes)
2. CSF evaluation for neurosyphilis: VDRL, protein (may be increased), cell count.
3. CBC (thrombocytopneia, anemia)
4. Liver function tests (elevated SGPT/SGOT, conjugated hyperbilirubinemia)
5. Ophthalmology consult (chorioretinitis)
At UTMB, the long bone films are the most likely part of the evaluation to be positive in the baby.
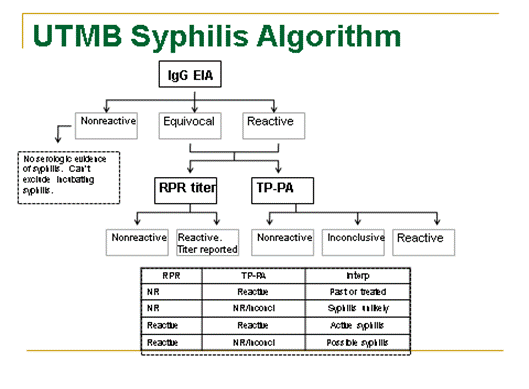
Management of the Asymptomatic Baby with Positive RPR and Mother with Positive RPR.
need to insert an image of this algorithm
Treatment of suspected congenital syphilis. For proven or probable congenital syphilis (based on the neonate's physical examination and radiographic and laboratory testing), the preferred treatment is aqueous crystalline penicillin G, administered intravenously. The dosage should be based on chronologic age rather than gestational age and is 50 000 units/kg IV, every 12 hours (1 week of age or younger) or every 8 hours (older than 1 week). Alternatively, procaine penicillin G, 50 000 units/kg, intramuscularly, can be administered as a single daily dose for 10 days; no treatment failures have occurred with this formulation despite its low CSF concentrations. When the neonate is at risk of congenital syphilis because of inadequate maternal treatment or response to treatment (or reinfection) during pregnancy but the neonate's physical examination, radiographic imaging, and laboratory analyses are normal (including infant RPR/VDRL either the same as or less than fourfold the maternal RPR/VDRL), some experts would treat with a single IM dose of benzathine penicillin G (Bicillin) at a dose of 50 000 units/kg. Most still would prefer 10 days of treatment. If more than 1 day of therapy is missed, the entire course should be restarted. Data supporting use of other antimicrobial agents (eg, ampicillin) for treatment of congenital syphilis are not available. When possible, a full 10-day course of penicillin is preferred, even if ampicillin initially was provided for possible sepsis. Use of agents other than penicillin requires close serologic follow-up to assess adequacy of therapy.
Infants who have a normal physical examination and a serum quantitative nontreponemal serologic titer either the same as or less than fourfold the maternal titer (eg, 1:4 is fourfold lower than 1:16) are at low risk of syphilis if (1) they are born to mothers who completed appropriate penicillin treatment for syphilis during pregnancy and more than 4 weeks before delivery; and (2) the mother had no evidence of reinfection or relapse. Although a full evaluation may be unnecessary, these infants should be treated with a single intramuscular injection of penicillin G benzathine, because fetal treatment failure can occur despite adequate maternal treatment during pregnancy. Alternatively, these infants may be examined carefully, preferably monthly, until their nontreponemal serologic test results are negative.
Minimal risk infants have a normal physical examination and a serum quantitative nontreponemal serologic titer either the same as or less than fourfold (eg, 1:4 is fourfold lower than 1:16) the maternal titer and (1) whose mother's treatment was adequate before pregnancy; and (2) whose mother's nontreponemal serologic titer remained low and stable before and during pregnancy and at delivery (VDRL less than 1:2; RPR less than 1:4) require no evaluation. Some experts, however, would treat with penicillin G benzathine as a single intramuscular injection if follow-up is uncertain.
Follow-up. All infants who have reactive serologic tests for syphilis or were born to mothers who were seroreactive at delivery should receive careful follow-up evaluations during regularly scheduled well-child care visits at 2, 4, 6, and 12 months of age. Serologic nontreponemal tests should be performed every 2 to 3 months until the nontreponemal test becomes nonreactive or the titer has decreased at least fourfold (eg, 1:16 to 1:4). Titers should decrease by 3 months of age and should be nonreactive by 6 months of age if the infant was infected and adequately treated or was not infected and initially seropositive because of transplacentally acquired maternal antibody. Patients with increasing titers or with persistent stable titers 6 to 12 months after initial treatment should be reevaluated, including a CSF examination, and treated with a 10-day course of parenteral penicillin G, even if they were treated previously.
Treponemal tests should not be used to evaluate treatment response, because passively transferred maternal treponemal antibodies can persist in an infant until 15 months of age. A reactive treponemal test after 18 months of age is diagnostic of congenital syphilis. If the nontreponemal test is nonreactive at this time, no further evaluation or treatment is necessary. If the nontreponemal test is reactive at 18 months of age, the infant should be evaluated (or reevaluated) fully and treated for congenital syphilis.
Treated infants with congenital neurosyphilis and initially positive results of VDRL tests of CSF or abnormal CSF cell counts and/or protein concentrations should undergo repeated clinical evaluation and CSF examination at 6-month intervals until their CSF examination is normal. A reactive result of VDRL testing of CSF at the 6-month interval is an indication for retreatment. Abnormal CSF indices that cannot be attributed to another ongoing illness also require retreatment. Neuroimaging studies, such as magnetic resonance imaging, should be considered in these children.
Tuberculosis
Management of Newborn Infant Whose Mother (or Other Household Contact) has Tuberculosis. Management of a newborn infant whose mother (or other household contact) is suspected of having tuberculosis is based on individual considerations. These infants should be reported to Hospital Epidemiology ASAP to assure home follow-up. If possible, separation of the mother (or contact) and infant should be minimized. The most commonly encountered circumstances are addressed below. Please see the Red Book for other situations:
Mother (or Other Household Contact) With a Positive Tuberculin Skin Test Reaction and No Evidence of Current Disease. A chest x-ray should be obtained on the mother. If mother has a negative CXR, no separation of mother and infant is necessary. Investigation of other members of the household or extended family to whom the infant may later be exposed is indicated. If no evidence of current disease is found in the mother or extended family, the infant should be tested with a Mantoux test (5 TU PPD) at 3 to 4 months of age should be done. When the family cannot be promptly tested, consideration should be given to the administration of INH (10 mg/kg/d) to the infant until skin testing of the family has excluded contact with a case of active tuberculosis. The infant does not need to be hospitalized during this time if adequate follow-up can be arranged. The mother is usually a candidate for INH preventive therapy.
Mother with Untreated (Newly Diagnosed) Disease or Disease Which Has Been Treated for 2 or More Weeks and Who Is Judged to Be Noncontagious at Delivery. Careful investigation of household members and extended family is mandatory. A chest roentgenogram and Mantoux tuberculin test should be done on the baby at 3 to 4 months and at 6 months. Separation of the mother and infant is not necessary if compliance with treatment for both mother and infant is assured. The mother can breast fed. The infant should receive INH even if the tuberculin skin test and chest roentgenogram do not suggest tuberculous disease, since cell-mediated immunity of a degree sufficient to mount a significant reaction to tuberculin skin testing may develop as late as age 6 months in an infant infected at birth. INH can be discontinued if the Mantoux skin test is negative at 3 to 4 months of age and no active disease exists in family members. The infant should be examined carefully at monthly intervals. If noncompliance is documented, the mother has AFB-positive sputum (or smear), and supervision is impossible, BCG (Bacillus Calmette-Guerin) vaccine may be considered for the infant. However, the response to the vaccine in infants may be inadequate for prevention of tuberculosis.
Mother Has Current Disease Suspected of Being Contagious at the Time of Delivery (abnormal CXR or evidence of active disease). The mother and infant should be separated until the mother is judged to be noncontagious, then proceed with management as in the preceding paragraph.
Varicella Zoster (Chickenpox)
Epidemiology is summarized as follows:
- Incubation period: 14-16 days (range 10-21 days); may be shorter in immunocompromised patients and longer (up to 28 days) in recipients of Varicella-Zoster immune globulin (VZIG).
- Contagiousness: most contagious 1-2 days before onset of the rash and for as long as 5 days after onset of lesions.
- Transmission: direct contact, airborne, intrauterine
- Immunity: generally lifelong, but reinfection can occur
Fetal Exposure in Early Gestation: The risk of Varicella embryopathy (congenital varicella) is 2%. Symptoms include scarring of the skin in a dermatomal distribution, limb atrophy with bone and muscle hypoplasia, ocular defects (cataracts, microphthalmia, chorioretinitis), and neurological abnormalities (mental retardation, microcephaly, dysfunction of bowel and bladder sphincters). Embryopathy does not require treatment with VZIG or isolation.
Fetal-Newborn Exposure in Late Gestation: If the mother develops varicella from 5 days before to 2 days after delivery, the newborn is at high risk of fulminant severe infection, with high mortality (30%). The baby develops varicella between 1 and 16 days of life (usual time from onset of rash in the mother to onset in the neonate is 9-15 days).
Treatment of the baby: Place in strict isolation, and if remains hospitalized, for up to 21 days (28 days if received VZIG). Administer VZIG, 125 units I.M. as soon as possible after delivery (for maximal effectiveness, give within 48 hours and not more than 96 hours after exposure). About 50% of babies who receive VZIG will develop varicella.
Postnatal Exposure, other than as discussed above, is not a problem for healthy term infants. VZIG is not indicated. These babies are at no greater risk for complications of chickenpox than are older infants and children. In the hospitalized population, VZIG is indicated for all preterm babies <28 weeks gestation or <1000g BW and for preterms >28 weeks gestation whose mothers have a negative history of infection. If an exposure occurs within the hospital, identify susceptible patients and give VZIG. The exposed personnel must follow airborne isolation procedures or stay away from work from 8-21 days after their exposure to varicella.
Isolation of the Hospitalized Patient. Airborne precautions must be followed. Establishment of the date of rash onset is key. For the index case, the patient must be isolated for 5 days from rash onset or duration of vesicular eruption (whichever is longer). Exposed patients must be isolated for 8-21 days after onset of rash in the index patient.
toc | return to top | previous page

